Anatomical challenges in TEVAR and recent developments in the design of thoracic aortic stent-grafts

| Available Online: | April, 2023 |
| Page: | 127-131 |
Author for correspondence:
Spyridon Mylonas
Department of Vascular and Endovascular Surgery, University of Cologne, Germany
E-mail: spyrosmylonas@gmail.com
doi: 10.59037/hjves.v4i4.8
ISSN 2732-7175 / 2022 Hellenic Society of Vascular and
Endovascular Surgery Published by Rotonda Publications All rights reserved. https://www.heljves.com
Spyridon Mylonas, MD, MSc, PhD, FEBVS1, Konstantinos Moulakakis, MD, MSc, PhD, FEBVS2
1 Department of Vascular and Endovascular Surgery, University of Cologne, Germany
2 Ass. Professor, Department of Vascular and Endovascular Surgery, University of Patras, Greece
Abstract
Full Text
References
Abstract
Abstract:
Endovascular repair is the treatment of choice for the patients suffering from thoracic aortic pathologies. Nevertheless, a higher proportion of young and female patients, as well as substantially high-profile delivery systems, lead to significantly higher morbidity rates or possibly the inability to implant any endoprosthesis at all. Thoracic stent-grafts have additional challenges to overcome due to the aortic curvature. This article will review the latest developments in thoracic stent-graft designs helped to address these anatomical challenges.
Full Text
INTRODUCTION
Thoracic endovascular aneurysm repair (TEVAR), is currently preferable over open surgery for the treatment of thoracic aortic pathologies.1 The rationale for this is the lower rates of perioperative morbidity and mortality associated with TEVAR when compared with open repair.2, 3 However, the feasibility, as well as, the short- and long-term clinical success of this procedure fundamentally depends on the treated anatomy and the ability of the stent-graft to be accommodated to this anatomy.
In a manner analogous to the implantation of stent-graft in the abdominal segment, anchoring in a sufficiently long healthy aortic segment proximal and distal to the pathology is required in order to achieve adequate apposition of the stentgraft, allowing a secure seal and fixation. Achieving a sealing zone of ≥ 2-cm centerline length is recommended.4 Moreover, an extremely angulated landing zone can lead to incomplete endograft apposition to the aortic lumen wall. A compromised landing zone increases the risk for endoleaks, bird-beaking configuration, retrograde aortic dissection, and even device migration or collapse.4, 5
igration or collapse.4, 5 Depending on the urgency, adjunctive procedures might be required to extend the landing zone and ensure a durable seal. These procedures include from the highly demanding total arch repair and the “frozen elephant trunk” procedure to other extra-anatomical arch debranching procedures, with less invasiveness (e.g transposition or bypass).6, 7 The proximal extent of the disease guides the proximal anchoring zone
and therefore, determines the extend of the repair. During the last decades stent-grafts with scallops, fenestrations or branches offer an alternative to hybrid procedures, whereas other authors also advocate the creation of in-situ fenestrations.8, 9 Moreover, the parallel graft technique or “chimney technique”, which involves deployment of stents/stent-grafts into the supra-aortic branches, with the proximal parts placed parallel to the main thoracic aortic stent-graft (between the aortic stent and the aortic wall) and extended above it to ensure perfusion, has also been used, with the advantage of immediate availability using off-the-shelf devices.10 Laser in situ arch fenestration is a further useful adjunct that has been used successfully in expanding the proximal zone of TEVAR to obtain adequate seal.11 Another issue that has to be dealt with is the morphology of the access vessels and the tortuosity of the aorta. It is well known that, among patients with descending thoracic aortic aneurysms female gender and young age are more common than among patients with abdominal aortic aneurysms. 2, 12 This population of patients has smaller access vessels, which can pose challenges in the advancement of the thoracic stentgraft while and increases the risk of access related complications. 13 MATERIAL AND METHODS
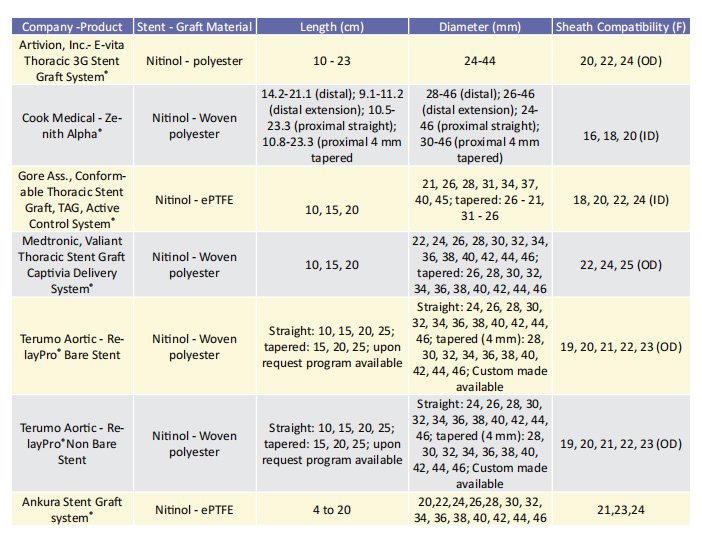
Aim of the present study is to list the advancements of the currently available in EU thoracic stent-grafts, which have been applied to address these anatomy-related challenges. The overview does not claim to cover all the available thoracic stent-grafts. However, it intends to illustrate the developments on the design and its impact on the results. Thus, the currently published studies on the new thoracic stent-grafts designs are presented.
Low-profile stent-grafts The delivery system has been reduced in size over the previous decade, which has been one of the most significant advancements in all aortic stent-grafts. This is especially important in the case of larger thoracic aortic stent-grafts, as delivery system diameter has been considered as a risk factor for morbidity and mortality.13 An overview of the available endografts with their characteristics, indicated for use in the descending thoracic aorta are included in table 1. oracic aorta are included in table 1. The Zenith Thoracic Alpha® (Cook Medical, Bloomington,IL, USA), the newer generation of the Zenith TX2® , was the first low-profile stent-graft developed for the thoracic aorta equipped with a built-in nose cone curvature (Pro Form® ) to improve conformability. Medium- and long-term outcomes of this device have been reported in a single-centre, retrospective study of 44 patients with a clinical follow-up period of at least 5 years.14, 15 The primary endpoint was continued clinical success (freedom from aneurysm-/procedure-related death, secondary intervention, type I or III endoleak, infection, thrombosis, aneurysm expansion, rupture, or conversion) according to the reporting standards for TEVAR.16 Secondary endpoints included stent fractures and fabric erosions, as well as migration of the prosthesis. Sustained clinical success was achieved in 84.1% of the patients. Four patients died in the postoperative course, whereas 3 type I or III endoleaks, and 1 aneurysm expansion without detectable endoleak were recorded. Graft-migrations were found in 2 patients (4.5%). No stent fracture was detected during the follow-up period while three patients (7%) had access vessel complications.
It was a great concern for many years whether reducing the size of delivery system would have an impact on durability. Both the metallic skeleton and the endograft fabric have
to be modified to fit within narrower introducers. In a study initiated by Cook, four migrations and one stent fracture were detected within a period of 12 months in a group of 110 patients with thoracic aortic aneurysms. To what extent these migrations could be attributed to the progression of aneurysmal degeneration in such a short time or if they were driven by the changes in design remained an unresolved topic. A second issue with the stent graft was the increased thrombus development within the device, which was described in two separate studies17, 18. In both cases, the stent graft had been implanted in a 24- and 29-year-old patient with blunt aortic trauma. The manufacturer then adjusted the Instructions for Use (IFU) and recommended use only for aneurysmal or ulcerative lesions.
The new generation of the Relay® platform (Relay® Pro) produced by Terumo Aortic (Inchinnan, UK), although based on the proven Relay® Plus design, offers a lower rofile (3-4 Freduction) through optimised weave pattern and radiopaque markers as well as thinner outer sheath. The performance of this design was evaluated in an international prospective multicenter single-arm study (The Regeneration Study). A total of 31 patients with thoracic descending aortic pathologies were enrolled. The technical success rate was 90%; three patients required a proximal extension due to intraoperative type Ia endoleak. The access vessel-related complication rate was 6%, with an average minimum diameter of 9.1 mm. During the follow up period of 12 months, there was 1 (3%) type Ib and 1 (3%) type II endoleak, recorded and 1 (3%) secondary intervention (to correct type Ib endoleak) was conducted.19 Another very intriguing endoprosthesis is the Valiant Navion® from Medtronic (Santa Rosa, CA, USA). Azzizadeh et al. also published the first results of this prosthesis in 201920. This report is interesting since it has more specific information on the access vessels. 71% of the patients had highly tortuous access vessels. 38% of the group (87 patients) were also female. In addition to achieving 100% technical success in the short term, access-related complication rate was 1%. Two patients died during the observation period of 30 days: In one case, retrograde type A dissection occurred, in the second case, a prosthesis infection led to rupture.
Similar to the COOK device concerns about the integrity of the stentgraft were raised. In the preliminary analysis of the imaging findings from the Valiant Evo Global Clinical Trial including 83 patients of the 100 patients originally enrolled in the trial, 11 patients with structural failures of the stentgraft were detected. Five patients had a type IIIb endoleak. In four of them the type III endoleak was associated with stent fractures consistent with the location of the graft seam and in one patient the type IIIb endoleak attributed to calcium erosion with no stent fracture or ring enlargement. Of the four patients with stent fracture in line with the graft seam, three underwent a relining procedure that successfully excluded the type IIIb endoleak. One of these three patients died 4 days later of suspected thoracic aortic rupture because the distal thoracic endovascular aortic repair extension had been landed in a previously dissected and fragile section of the aorta. The remaining six patients had had stent ring enlargement. One of the six patients had had persistent aneurysm expansion from the time of implantation onward and had died of unknown causes.21 These findings led FDA at September 2021 to order a Class I recall of the device.
Improved release and adaptation to aortic morphology For aneurysms involving the aortic arch, endovascular treatment is more challenging than for those in the abdominal region. If the wall apposition of the endograft to the aorta is insufficient, the bird-beak effect occurs with a consecutively increased risk of a type I endoleak.5 Stent-grafts of earlier generations were more prone to bird-beaking because the proximal part could not completely conform to the aortic anatomy, with an incidence as high as 40% to 57%. 22 Other associated complications such as collapse of the endoprosthesis23 or migration during implantation5 have also been described. Therefore, several attempts have been undertaken by the manufacturers to optimize the apposition of thoracic endografts to the curvature of the aorta. 24 Moreover, any unintentional covering of the supraaortic ostia by the stentgraft or through manipulation in the aortic arch might result in a cerebral insult. Thus, accurate alignment of the graft in this aortic segment is of particular importance. However, the more demanding hemodynamics in aortic arch compared to other aortic segments make accurate release more challenging. Even with the stentgraft perfectly positioned, deployment of the graft can be complicated secondary to the high volume of blood flow in the thoracic aorta resulting in the “windsock effect,” which describes the tendency of the graft to be migrated distally before deployment is complete. This is especially true with deployment mechanisms where the proximal end opens while the distal end remains constrained. Although several maneuvers have been suggested to reduce the cardiac output during the deployment the risk of distal migration is not completed eliminated.25, 26 The Gore Conformable TAG Stent Graft with ACTIVE CONTROL System® (CTAG, Gore Medical, Flagstaff, AZ, USA) was specially designed to overcome this problem. In particular, this new version of the graft introduces novel features that help to enhance deployment accuracy and stent graft apposition and to fully take advantage of the stentgraft’s conformability. First, the in situ post- deployment curving ability, which allows the proximal part of stents to be curved to fit the aortic arch achieving high apposition. Secondly the two-step release mechanism: In the first step the endograft opens from proximal to distal along its entire length to its intermediate diameter, which is approximately 50% of its nominal diameter. Importantly this staged deployment reduces the wind-socking forces while repositioning is easily possible. In the second step, after ensuring precise positioning, full release of the stentgraft from the middle to the ends can follow.
Aiming to evaluate these theoretical advantages 127 patients who were treated with the CTAG with Active control were enrolled in a prospective, multi-center study between
October 2017 and July 2018.27 The primary endpoint was technical success. Secondary endpoints included clinical success and major adverse events at 30 days and 12 months. In addition, the frequency and the reasons of use of the aforementioned mechanisms were recorded. The primary endpoint was met in 124 patients (97.6%). In 3 patients the ostium of the left common carotid artery was unintentionally partially covered. In all of these patients, the landing zone was significantly shorter than 20 mm. There were 3 aorta-related deaths (due to retrograde aortic dissection, spinal cord ischemia and bowel ischemia) within 30 days and 3 further within 12 months postoperatively resulting in a 30-day clinical success rate of 97.6% and a 12-month clinical success rate of 92.9%.
The angulation feature was applied in 64 cases (50.4%) and the desired effect was achieved in 60 cases (93.8%). Rapid ventricular pacing during deployment was used only in 9 procedures (7.1%). There were no reports of device compression, bird-beak configuration, fracture, or graft occlusion. During follow-up, there were 2 type Ia endoleaks (1.6%); 1 was due to stent-graft migration (0.8%). The access-related complication rate was 2.4% Another interesting finding, is that the cTAG endograft with active control has a significantly lower stroke rate (0.8% vs. 11%) compared to the previous model.28 A possible explanation is that owing to the above mentioned mechanism fewer manipulations of the stentgraft in the aortic arch as well as less frequent need for cardiac output reduction are required and thus the risk of embolism is reduced.
Recently two new stentgrafts have been initiated equipped with unique features intending to broaden the spectrum of patients can be treated endovascularly. The Ankura Stent Graft system® (Lifetech Scientific, Shenzhen, China), which consists of a dual-layer expanded polytetrafluoroethylene membrane, without suture on the main body and a nitinol skeleton with asymmetric wave design and the proximal mini-wave stent. In a single center retrospective study on 30 patients a technical success rate of 97% was reported; in one patient the deployment of the device could not be completed due to the extreme tortuosity of the descending thoracic aorta. During the first 30 days after the procedure two patients (6.8%) died (due to gastrointestinal bleeding and sepsis after pulmonary infection). Two (7%) access site complications were recorded. During a median follow-up of 31.7 (range, 38.4) months, two more patients died of non-TEVAR-related causes and two patients (7%) developed type Ia endoleak.29 nts (7%) developed type Ia endoleak.29 The Cryolife E-nya ® thoracic stentgraft system, which recently receives CE mark. It is constructed of lower profile graft material and offers both bare spring and covered proximal configurations with tip capture technology, enhancing the control and predictability during deployment. Initial results with this stent-graft are to be published.
Limitations
Currently there are no randomized trials, which directly compare the results of the available thoracic stent-grafts. On the other hand, a direct comparison between the thoracic stentrafts in terms of El type Ia would not be justified as the the risk for type Ia EL is associated with the intended proximal landing zone, with very low rates being more likely to be achieved when treating lesions in the descending thoracic aorta. 20, 30 Aortic curvature in zone 4 is less prominent, bird-beaking is less common in this zone. On the contrary landing in more proximal zones tends to be associated with type Ia endoleak rates of around 4%.15, 19, 31 Moreover, because the intended landing zone is not defined with millimeter precision, but rather only very generally, as the placement at the “desired location” is provided in the published studies, a comparison among the available stentgrafts is not possible. This limitation underlines the importance of adopting more accurate reporting standards to determine the precise deployment and the wall apposition.32
CONCLUSION AND OUTLOOK
All of the major manufacturers of thoracic stentgrafts have introduced new, significantly improved grafts in the last 5 to 10 years, which have been shown to be able to be used in more patients with more difficult access vessels and become more effective in dealing with challenging anatomy. In the future, further development will focus on the ascending aorta and the proximal aortic arch segments in order to be able to treat ascending and aortic arch pathologies safely and reliably using endovascular treatment.
References
1 Rabe, E., Régnier, C., Goron, F., Salmat, G. & Pannier, F. The prevalence, disease characteristics and treatment of chronic venous disease: An international web-based survey. J. Comp. Eff. Res. 9, 1205-1218 (2020)
2 Lurie F, Passman M, Meisner M, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord 2020;8:342-352
3 Salim, S., Machin, M., Patterson, B. O., Onida, S. & Davies, A. H. Global Epidemiology of Chronic Venous Disease. Ann. Surg. 274, 971-976 (2021)
4 Zolotukhin, I. A. et al. Prevalence and Risk Factors for Chronic Venous Disease in the General Russian Population. Eur. J. Vasc. Endovasc. Surg. 54, 752-758 (2017)
5 Bartolo M. Socioeconomic impact of venous diseases in Italy. Phlebologie 1992; 45: 423-31.
6 Canonico S, Gallo C, Paolisso G, Pacifico F, Signoriello G, Sciaudone G, et al. Prevalence of varicose veins in an Italian elderly population. Angiology 1998; 49: 129-35.
7 Carpentier, P. H., Maricq, H. R., Biro, C., Ponçot-Makinen, C. O. & Franco, A. Prevalence, risk factors, and clinical patterns of chronic venous disorders of lower limbs: A population-based study in France. J. Vasc. Surg. 40, 650-659 (2004)
8 Fowkes FGR, Evans CJ, Lee AJ. Prevalence and risk factors of chronic venous insufficiency. Angiology 2001; 52: S5-S15.
9 Dimakakos, E., Syrigos, K., Scliros, E. & Karaitianos, I. Prevalence, risk and aggravating factors of chronic venous disease: An epidemiological survey of the general population of Greece. Phlebology 28, 184-190 (2013)
10 Liapis H, Papavasiliou V. Prevalence of venous diseases in the Greek population. In: Proceedings of the 22nd Annual Hellenic Medical Conference. Hellenic Medical Association, Athens 1996, 9-16.
11 Lionis, C. et al. Chronic venous insufficiency. A common health problem in general practice in Greece. Int. Angiol. 21, 86-92 (2002)
12 Launois R, Reboul-Marty J, Henry B. Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Qual Life Res.
1996;5:539-554.
13 Le Moine JG, Fiestas-Navarrete L, Katumba K, Launois R. Psychometric Validation of the 14 items ChronIc Venous Insufficiency Quality of Life Questionnaire (CIVIQ-14):
Confirmatory Factor Analysis. Eur J Vasc Endovasc Surg. 2016 Feb;51(2):268-74.
14 Erevnidou K, Launois R, Katsamouris A, Lionis C. Translation and validation of a quality of life questionnaire for chronic lower limb venous insufficiency into Greek. Int
Angiol. 2004;23(4):394-399.
15 Lwanga, Stephen Kaggwa, Lemeshow, Stanley & World Health Organization. (1991). Sample size determination in health studies: a practical manual / S. K. Lwanga and S.
Lemeshow. World Health Organization. https://apps.who. int/iris/handle/10665/40062


