Prevalence of Chronic Venous Insufficiency and Quality of Life in the Greek Population: Protocol and Study Design

| Available Online: | Desember, 2022 |
| Page: | 122-126 |
Author for correspondence:
Efthymios Avgerinos
Vascular and Endovascular Clinic, Athens Medical Center Kifisias 56 & Delfon, 151 25 Marousi, Athens, Greece
Τel: +30 210 6862637 Μ: +30 6944 570086
E-mail: eavgerinos@vascularhealth.gr
doi: 10.59037/hjves.v4i4.7
ISSN 2732-7175 / 2022 Hellenic Society of Vascular and Endovascular Surgery Published by Rotonda Publications
All rights reserved. https://www.heljves.com
Efthymios D. Avgerinos1,2, Spyros N. Vasdekis1 , Dimitrios G. Kardoulas1 , Garyfallia Stefanou3 , Georgia Kourlaba4, Antonios Papageorgiou5
1 Department of Vascular Surgery, Attikon Hospital, University of Athens
2 Clinic of Vascular and Endovascular Surgery, Athens Medical Center
3 ECONCARE LP, Athens, Greece
4 Department of Nursing, University of Peloponnese
5 Department of Vascular Surgery, Athens, Navy Hospital Under the auspices of the Hellenic Society of Phlebology
Abstract
Full Text
References
Abstract
Chronic venous insufficiency (CVI) is very common in the western world with various studies reporting a prevalence of 46-84%, depending on the population studied, the definition of CVI and the mode of diagnosis (ultrasound or clinical). In Greece, a few studies have focused on this chronic health problem and the findings are contradictory in terms of CVI incidence in the two genders, in different age groups and in urban or rural areas. We herein present the protocol and study design of our study targeting to assess the prevalence of CVI in Greece and the quality of life of patients at different stages of the disease (C0s-C6).
A cross-sectional study will be carried out in Greece between December 2022 and February 2023. The study will include ~2,300 adults constituting a random and representative sample of the general population in terms of geographic region of residence and gender based on the most recent census (2021). This sample will be selected from a random sample of pharmacies invited to participate in the study per geographic region; this will be proportional to the number of in dividuals to be included per geographic region to ensure representativeness. Each pharmacy will enroll 10 individuals.
Data collection will be carried out with the help of a questionnaire focusing on CVI symptoms. Colour photographs of lower extremities of different CVI stages will help the respondents to select whether they identify with one of these stages (C0s-C6). Their quality of life will be assessed via the CIVIQ-14 questionnaire.
Full Text
Chronic venous insufficiency (CVI) of the lower extremities is a particularly common condition affecting the general population and includes a wide range of signs and symptoms that impact patient health and quality of life. The underlying cause is venous hypertension due to structural or functional abnor malities of the veins (venous insufficiency). The causal basis of CVI is multifactorial and is partly due to hereditary predisposition, partly due to lifestyle or other factors (e.g., pregnancy, history of thrombosis). Regardless of its causal basis, CVI has significant socio-economic consequences and significantly affects patients’ quality of life. 1Although CVI may be asymptomatic in the very early stages, symptoms develop gradually and are increasingly worsen with age. Common symptoms are heaviness or pain, swelling, muscle cramps, burning sensation, itching sensation and restless legs. The physical signs include the appearance of telangiectasias in the initial stages, followed by venous varicosities and edema, and in the advanced stages it manifests as skin discoloration and ulcers that are difficult to heal. Due to the chronic nature of the disease, exacerbations and remissions often occur in combination with the influence of environmental factors. The clinical staging of CVI is described in the CEAP classification (Clinical condition, Etiology, Anatomic distribution and Pathophysiology). 2 In daily clinical practice, we limit ourselves to the “C”, clinical condition:
− 0: No visible or palpable signs of venous disease
− 0s: No visible or palpable veins, without signs but with symptoms of venous disease (this subcategory has been eliminated in the 2020 revised classification)
− Ι: Telangiectasias, reticular veins
− ΙΙ: Varicose veins
− IIr: Recurrent varicose veins
− ΙΙΙ: Edema without skin lesions
− IV: Changes in and subcutaneous tissue secondary to chronic venous disease
– IVa: Pigmentation or eczema
− IVb: Lipodermatosclerosis or atrophie blanche
− IVc: Corona phlebectatica
− V: Skin lesions as above with a healed ulcer
− VI: Skin lesions as above with an active ulcer
− VIr: Recurrent active venous ulcer
CVI is very common in the western world with various studies reporting a prevalence of 46-84%, depending on the population/sample, the exact definition of CVI (C0s-C6, C1-C6 etc.) and the mode of diagnosis (ultrasound or clinical).1-8 Given the progressive aging of the population, the frequency is expected to increase. In Greece, a few studies have focused on this chronic health problem and the findings are contradictory in terms of CVI incidence in the two genders, in differentage groups and in urban or rural areas. 9-11 The primary objective of our study is to assess the prevalence of CVI in Greece and the quality of life of patients at different stages of the disease (C0s-C6). We herein present our study protocol and design.
METHODOLOGY
Study design
In order to serve the primary objective of this study, a cross-sectional study will be carried out in Greece between December 2022 and February 2023. The study will include ~2,300 adults constituting a random and representative sample of the general population in terms of geographic region of residence and gender based on the most recent census (2021). This sample will be selected from a random sample of pharmacies invited to participate in the study per geographic region; this will be proportional to the number of individuals to be included per geographic region to ensure representativeness, given that each pharmacy will enroll 10 individuals. The pharmacists who will accept to participate in the study will attend a dedicated training session for the needs of data collection. Specifically, patients eligible for inclusion in the study will be people over 18 years of age, inhabitants of the respective geographic region, who will visit the study pharmacies between 10:00 am and 12:00 pm.
In order to maintain gender representativeness of the overall Greek population in the sample, pharmacists will not target individuals of the gender for which the number of individuals has been completed, based on initial estimates. Each pharmacy will stop collecting data once they have completed the required number of questionnaires for men and women (5 questionnaires from each gender). In addition to the questionnaires, each pharmacy will record the number of people it addressed in total during the collection in order to estimate the response rate to the study. Table 1 shows the distribution of participants by gender and geographic region. From each geographic region, one or more cities will be selected that are considered representative of the region (one of them will be its seat). In the case of more than one city selected per geographic region, the distribution of pharmacies will be proportional to the number of inhabitnts of the city. The number of pharmacies – study sites percity is listed in Table
2.Data collection
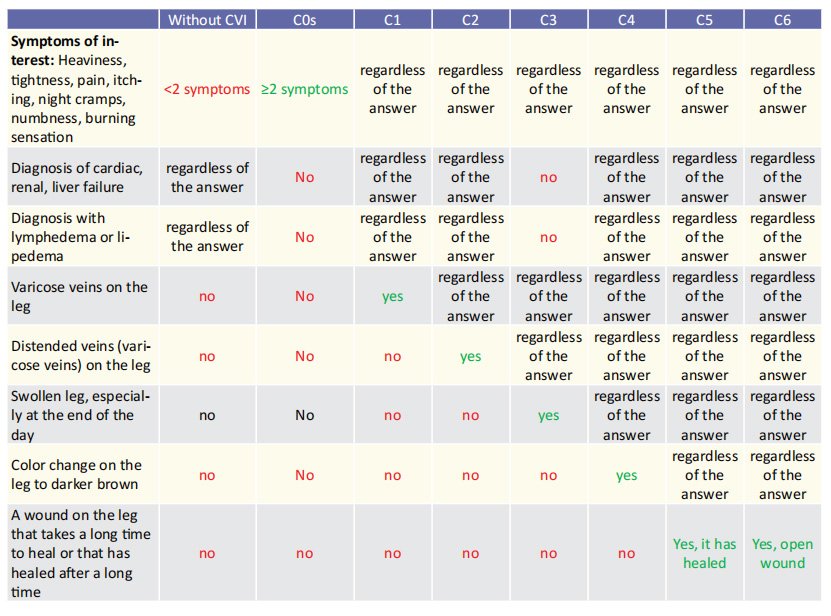
Data collection, performed at pharmacies, will be carried out with the help of a questionnaire designed to serve the objectives of this study. This questionnaire includes questions about participants’ general demographic data (geographic region, age, gender, height, weight), lifestyle (daily activity, smoking), family history of venous disease and personal medical history (venous thrombosis for which the participant received anticoagulation, heart/kidney/liver failure, diagnosis with lymphedema or lipedema) and focus on symptoms and signs suggestive of venous insufficiency. Colour photographs of lower extremities of different CVI stages will help the respondents to select whether they identify with one of these stages (C0s-C6). Part of the questionnaire will be the CIVIQ-14 questionnaire.12,13 The estimation of CVI prevalence by stage (C0s-C6) will be based on patient responses. The staging will be based on the rule presented in Table 3. Participants diagnosed with any stage (C0s-C6) will also be asked about their use of elastic compression stockings, treatment for symptoms of venous insufficiency in the legs, any possible kind of vein intervention, and their quality of life via the CIVIQ-14 questionnaire. The CIVIQ questionnaire has been translated and adjusted to Greek. 14
Prevalence of Chronic Venous Insufficiency and Quality of Life in the Greek Population: Protocol and Study Design
Table 1. Distribution of participants by gender and geographic region
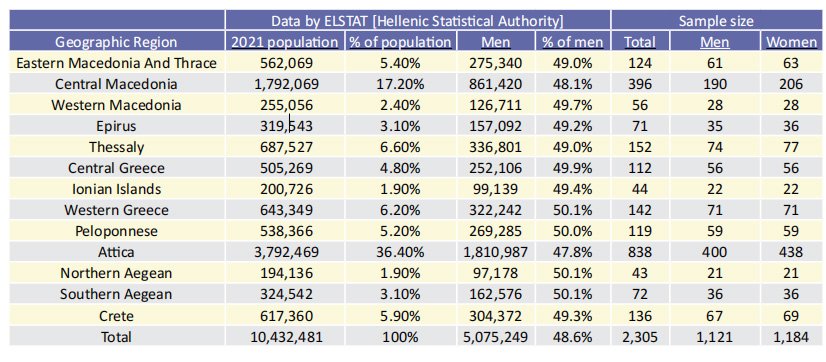
Table 2. Identification of cities and number of pharmacies from each city

Table 3. Staging rule based on symptoms, diagnoses and photographs of lower extremities
STATISTICAL ANALYSIS
Calculation of the sample size
The sample size estimate was based on the assumption that the prevalence of venous insufficiency (C0s-C6) would be about 60%, based on the available literature.1,3,9,10 Therefore, with a statistical significance level of 5% (a) and an absolute precision (d) equal to 2%, the resulting sample size is 2,305 individuals using Lwanga’s and Lemeshow’s equation. 15Data analysis The study data will be further weighted for the age distribution of the population by sex and geographic region as derived from the latest ELSTAT census (2011 or 2021, whichever is available). The results of the study will be presented with appropriate descriptive means: qualitative variables with absolute (n) and relative frequencies (%), and quantitative variables with mean and standard deviation (SD) or median and 1st – 3rd quartile (Q1 – Q3). Prevalence will be reported with percentages (%) and 95% confidence intervals (CIs). Correlations between two continuous variables will be tested with Pearson’s or Spearman’s rho, between two categorical variables with Pearson’s Chi-square test or Fisher’s exact test, and between a categorical and a continuous variable with Student’s t -test or the Mann – Whitney U test. In addition, generalized linear models will be applied by selecting an appropriate distribution from the family of exponential distributions and an appropriate link function to investigate demographic and clinical factors associated with venous insuf ficiency, its severity and the patients’ quality of life of patients. All tests will consider the weights of the observations. All tests will be performed at an α = 5% significance lev Data processing and statistical analyses will be carried out with the statistical program Stata 17.
RESEARCH ETHICS
In conducting this research, all personal data security proocols are to be followed and the confidentiality of the data collected from the sample is to be preserved. In the database that will be created, the data will be anonymized. This study will be conducted in accordance with the principles underlying the Declaration of Helsinki, thus ensuring compliance with regulatory standards that guarantee that the safety, rights and welfare of the participants involved in this study are protected. Written informed consent will be obtained for any individual to participate in the study. At the same time, permission to approve the research protocol will be requested from the ethics committee of the Navy Hospital of Athens (NNA), while it is also worth mentioning that the data collected from the questionnaire responses will be used exclusively for the completion of this research and not for any other purpose.
CONFLICTS OF INTEREST
The study is sponsored by the pharmaceutical company Servier Hellas Pharmaceutique E.P.E.
References
1 he prevalence, disease characteristics and treatment of chronic venous disease: An international web-based survey. J. Comp. Eff. Res. 9, 1205-1218 (2020)
2 Lurie F, Passman M, Meisner M, et al. The 2020 update of the CEAP classification system and reporting standards. JVasc Surg Venous Lymphat Disord 2020;8:342-352
3 Salim, S., Machin, M., Patterson, B. O., Onida, S. & Davies, H. Global Epidemiology of Chronic Venous Disease. Ann. Surg. 274, 971-976 (2021)
4 Zolotukhin, I. A. et al. Prevalence and Risk Factors for Chronic Venous Disease in the General Russian Population. Eur. J. Vasc. Endovasc. Surg. 54, 752-758 (2017)
5 Bartolo M. Socioeconomic impact of venous diseases in Italy. Phlebologie 1992; 45: 423-31.
6 Canonico S, Gallo C, Paolisso G, Pacifico F, Signoriello G, Sciaudone G, et al. Prevalence of varicose veins in an Italian elderly population. Angiology 1998; 49: 129-35.
7 Carpentier, P. H., Maricq, H. R., Biro, C., Ponçot-Makinen, O. & Franco, A. Prevalence, risk factors, and clinical patterns of chronic venous disorders of lower limbs: A population-based study in France. J. Vasc. Surg. 40, 650-659 (2004)
8 Fowkes FGR, Evans CJ, Lee AJ. Prevalence and risk factors of chronic venous insufficiency. Angiology 2001; 52: S5-S15.
9 Dimakakos, E., Syrigos, K., Scliros, E. & Karaitianos, I. Prevalence, risk and aggravating factors of chronic venous disease: An epidemiological survey of the general population of Greece. Phlebology 28, 184-190 (2013)
10 Liapis H, Papavasiliou V. Prevalence of venous diseases in the Greek population. In: Proceedings of the 22nd Annual Hellenic Medical Conference. Hellenic Medical ssociation, Athens 1996, 9-16.
11 Lionis, C. et al. Chronic venous insufficiency. A common health problem in general practice in Greece. Int. Angiol. 21, 86-92 (2002)
12 Launois R, Reboul-Marty J, Henry B. Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Qual Life Res. 1996;5:539-554.
13 Le Moine JG, Fiestas-Navarrete L, Katumba K, Launois R. Psychometric Validation of the 14 items ChronIc Venous Insufficiency Quality of Life Questionnaire (CIVIQ-14): Confirmatory Factor Analysis. Eur J Vasc Endovasc Surg. 2016 Feb;51(2):268-74.
14 Erevnidou K, Launois R, Katsamouris A, Lionis C. Translation and validation of a quality of life questionnaire for chronic lower limb venous insufficiency into Greek. Int Angiol. 2004;23(4):394-399.
15 Lwanga, Stephen Kaggwa, Lemeshow, Stanley & World Health Organization. (1991). Sample size determination in health studies: a practical manual / S. K. Lwanga and S. Lemeshow. World Health Organization. https://apps.who. int/iris/handle/10665/40062


