Submission
All manuscripts are submitted through the official site of the
Journal: heljves.com, https://heljves.com/Submit-Paper/
Submission list:
- Cover letter (word format)
- Manuscript [word format; includes: abstract, main manuscript, table legends, tables, figure legends and figures (optional)]
- Figures (Tiff format; more details in figure files section)
Instructions for authors in detail
Overview
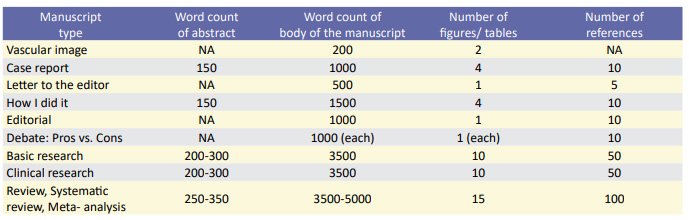
Manuscripts considered for publication must be written in English and structured as a basic or clinical research, editorial, case report, vascular images, letter to the editors, debate with with pros and cons, technical description on ‘how I did it’, review, systematic review and meta-analysis. Type of articles and their word count, table, figure and reference numbers demonstrated in table 1.
Peer Review
All manuscripts are reviewed initially by the Chief Editor, Executive Editor, and/or their representatives. A submission is rejected outright if the majority opinion finds that (1) the material does not have sufficient merit to warrant further review or (2) the subject matter is outside the scope of the Journal. The submission may also be returned for inadequate presentation or failure to comply with the HJVES’s submission instructions. Authors should read the HJVES Editorial Policies before constructing the manuscript; it contains detailed information about originality, authorship, primacy, research and animal experimentation, patient consent, conflict of interest disclosure, funding, permissions, scientific misconduct, peer review, contributor publishing agreement, and publication.
Cover Letter
Manuscripts (except correspondence) must be accompanied by a cover letter signed by all authors stating (1) there has been no duplicate publication or submission of any part of the work; (2) all authors have read and approved the manuscript; and (3) there is no financial arrangement or other relationship that could be construed as a conflict of interest. If a potentialmconflict exists, its nature should be stated in the letter and on the title page for each author involved (see Editorial Policies for a complete explanation).
Title Page File
Construct a title that does not exceed 50 words. Z List first and last names, highest academic degree(s), affiliations, and email addresses for all authors. Z Give the total word count. Z Acknowledge all sources of financial support (grants, fellowships, equipment, or remuneration of any kind) and any relationships that may be considered a conflict of interest (ie, employment, stock holdings, retainers, paid or unpaid consultancies, patents or patent licensing arrangements, or honoraria) that may pertain to the manuscript (see Editorial Policies, Conflict of Interest). Z Give details of any prior presentation, including meeting name, location, and date.
References
- Follow the guidelines in the AMA Manual of Style (10th ed., 2007). Do NOT use
endnotes or other bibliographic style function for reference lists. - Limit to 10 the number of references for a case report/ ‘how I did it’/ editorial and
debate with pros and cons. Letters may have no more than 5 references. Basic and clinical articles may have up to 50 references, while systematic review and meta-analysis up to 100 references. - Number references in the order of appearance in the text. Identify references in the text, tables, and legends as superscript Arabic numerals.
- List the first 6 authors (last name and initials separated by a comma); use “et al” for 6 or more authors. Abbreviate journal titles according to the style of Index Medicus; formats for the common types of journal citations are: Journal article: authors’ names and initials, article title, journal name, year, volume, and inclusive page numbers.
Examples:
- Type all figure and table legends on a separate page of the manuscript file, explaining abbreviations and symbols used in the figure. Previously published figures must be acknowledged and accompanied by written permission from the publisher to reproduce the material if it is copyrighted.
- Do not use Word’s caption function for figure legends or include the actual figures in the manuscript file.
Table Files:
- Use tables to supplement the text, not duplicate it.
- Insert tables in the text or create/save tables as an image.
- Format tables using the table formatting function in Word; elaborate formatting
(shading, color) should not be used. - Define any abbreviations as the first footnote under the table; list the abbreviations alphabetically.
- Use footnotes for explanatory material, labeling each with a superscript lower
case letter (a-z) in alphabetical order.
List acknowledgments, any shared first authorship, and other author notes.
Z Give the name, mailing address, and email address of the corresponding author.
Abstract
Give a substantive summary of a basic or clinical research article in 300 words or less, and up to 350 words for systematic reviews and meta-analysis, separating the abstract according to Introduction, Methods, Results, and Conclusion. For case reports and ‘how I did it’ the abstract should be no longer than 150 words and divided into Purpose, Case Report/ Technique and Conclusion.
Provide up to 5 keywords.
Text
- Text material must be submitted as a single Word document (not a PDF) named the “Main document.”
- Organize the text for clinical or basic experimental investigations and systematic review and meta-analysis into sections entitled Introduction, Methods, Results, Discussion, and Conclusion. Case reports and ‘How I did it’ require only Introduction, Case Report/Technique, Discussion, and Conclusion. Editorials, letter to the editor, vascular images, and debate with pros and cons may be structured as as appropriate
for the material. - Avoid naming any institution(s) in the work or otherwise identifying the author(s).
- Use Sl measurements; generic drug names should be used.
- Define abbreviations and acronyms when they first appear in the text; do not list
them at the beginning of the manuscript file. - Identify tables and figures using Arabic numerals in parentheses (eg, Table 1,
Figure 1).
Figure Files
- Number any pictures, charts, graphs, or line art sequentially as figures.
- Use color judiciously in pictures and graphics. Figures will be printed in grayscale unless color charges are paid. The fees for color are ….euro for the first figure and …euro for each additional color figure for print/online display. Color figures will appear in the digital version at no charge.
- Add arrows and symbols to digitally created images using functions supplied with the imaging program.
- Do not import images into the text document but transmit each image file
separately. - Supply all figures in a digital format of suitable quality for printing: TIF for pictures or EPS for graphs and line drawings (to preserve quality when enlarged/zoomed). Image resolution should be at least 300 ppi for color or grayscale images and 600 ppi (preferably higher) for black and white line drawings or graphs. Image size at these resolutions should be no less than 3 inches wide for vertical images and 5 inches
wide for horizontally oriented figures. Use a lossless compression algorithm (eg, LZW) that does not degrade the resolution. - Convert PowerPoint slides to individual TIF files for upload.
Publication
Accepted manuscripts will be scheduled for publication generally in the order in which they are received after no further author revisions are required and the Journal Contributor’s Publishing Agreement has been signed by the corresponding author. The HelJVES reserves the right to edit accepted manuscripts to comply with the journal’s format, to correct grammatical faults, to remove redundancies, and to improve readability without altering the meaning. Several weeks before the scheduled publication of an article, the Editorial Office will send via email an edited version of the manuscript to the corresponding author for approval. After the author has approved the edited version, the publisher will send a PDF of the page proof by email. At this stage, only correction of typographical errors or mistakes in the presentation of data can be made. Approval/changes to the proof must be returned within 2 days. The HelJVES value their relationships with their authors and appreciate author compliance with these instructions.
Hellenic Journal of Vascular and Endovascular Surgery (HJVES) Editorial Policies
Originality
Materials submitted to the Hellenic Journal of Vascular and Endovascular Surgery (HelJVES) must be original; they cannot have been previously published (other than as abstracts) nor can they be under simultaneous consideration by any other journal. If the work has been presented at a meeting or has been published as an abstract, a statement to this effect must appear in Authors’ Notes on the title page, identifying the meeting, location, and date or details of the abstract publication. In general, manuscripts will not be considered if the work has been published in full-length conference proceedings or as a book chapter. Primacy The HelJVES does not allow overt claims to first conceptualization, identification, or use of any device, technique, or treatment; however, an effort is made to convey the potential of initial publication if appropriate.
Authorship Responsibility
Articles submitted for consideration require consent by all contributing authors. The submitting author or author’s representative should carefully check that all individuals who contributeed to the article are listed as authors.
Authors are those who:
- made a substantial contribution to the concept and design, acquisition of data, oranalysis and interpretation of data;
- draftedthe article or revised it critically for important intellectual content; ¨approved the version to be published; and
- agreed to be accountable for all aspects of the work, ensuring that questionsrelated to the accuracy or integrity of any part of the work are appropriately investigated and
Please refer to the International Committee of Medical Journal Editors (ICMJE) authorship guidelines. Any contributors who do not meet the criteria for authorship should be listed in “Acknowledgements” on the title page. Examples of those who might be acknowledged include a person who provided purely technical help, writing assistance, or a department chairperson who provided only general support. Authors should disclose any writing assistance and identify the entity that paid for this assistance.
Research and Animal Experimentation
Human and/or animal studies must be conducted to a high ethical standard. Studies involving human subjects should conform to the Declaration of Helsinki. Care of experimental animals must follow the Guide for the Care and Use of Laboratory Animals or similar national guidelines. Studies involving human subjects or animal models must have approval of the Institutional Review Board (IRB) or other agency responsible for human and/or animal research at the authors’ institution(s).
Statements certifying compliance with these requirements for human subjects and animals must appear in the Methods section of the manuscript. The authors must provide the full name of the review committee and institution and an Ethics Committee reference number. In line with the Declaration of Helsinki 1975, revised Hong Kong 1989, authors are encouraged to register their clinical trials [at ClinicalTrials.gov or other suitable databases identified by the ICMJE]. The registration information for a trial should be stated in the abstract and at first mention of the study in the body of the manuscript.
Patient Consent
Authors are required to follow the ICMJE guidelines Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals. Human subjects must have given written informed consent to participate in any study or undergo any procedure considered investigational or experimental in a document approved by the IRB or similar governing body. Patients have a right to privacy that should not be infringed without informed consent. Identifying information, including patients’ names, initials, or hospital numbers, should not be published in written descriptions or photographs unless the information is essential for scientific purposes and the patient (or parent or guardian) gives written informed consent for publication. Informed consent for this purpose requires that a patient who is identifiable be shown the manuscript to be published. Identifying details should be omitted if they are not essential. Complete anonymity is difficult to achieve, however, and informed consent should be obtained if there is any doubt.
For example, masking the eye region in photographs of patients is inadequate protection of anonymity. If identifying characteristics are altered to protect anonymity, authors should provide assurance that alterations do not distort scientific meaning and the editors should so note. When informed consent has been obtained, it should be indicated in the article.
Conflict of Interest Disclosure
Authors are obliged to disclose in the submission letter and the manuscript any financial arrangement or other relationship that could be construed as a conflict of interest for any product mentioned in the manuscript. Position your declaration on the title page, under a heading “Declaration of Conflict of interests.” If no declaration is made, the following will be printed under this heading in your article: “The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.” When making a declaration, the disclosure information must be specific and include any financial relationship that any author of the article has with any sponsoring organization and the forprofit interests the organization represents and with any for-profit product discussed or implied in the text of the article. These relationships include but are not limited to employment, direct payments, stock holdings, retainers, paid or unpaid consultancies, patents or patent licensing arrangements, research funding, speakers’ bureau, or honoraria with any individual, company, or organization having a vested interest in the subject matter or products mentioned in the manuscript. For more information please visit the Journal Author Gateway. If the Chief Editor believes or is notified that an author may have failed to make an appropriate disclosure, the author will be contacted. Depending upon the response, the Editor will decide if a corrigendum correcting the oversight should be published or if more serious action is warranted in the case of deception. In that event, the Editor may publish a notice that the author did not comply with the HelJVES’s requirement to disclose a conflict of interest, calling into question the reliability of the article.
Funding
All authors are required to acknowledge their funding in a consistent fashion under a separate heading. Please visit Funding Acknowledgements on the Journal Author Gateway to confirm the format of the acknowledgment text in the event of funding or state in the acknowledgments that: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Permissions Authors are responsible for obtaining permission from copyright holders for reproducing any illustrations, tables, figures, or lengthy quotations previously published elsewhere. For further information, including guidance on fair dealing with criticism and review, please visit the Frequently Asked Questions on the Journal Author Gateway.
Scientific Misconduct
The HelJVES takes issues of copyright infringement, plagiarism, or other breaches of best practice in publication very seriously. Every effort is made to protect the rights of authors, and claims of plagiarism or misuse of articles published in the journal are always investigated. Equally, the reputetion of the journal is protected against malpractice. Submitted articles may be checked using duplication- checking software. Where an article is found to have plagiarized other work or included thirdparty copyright material without permission or with insufficient acknowledgment, or where authorship of the article is contested, the publisher reserves the right to take action including but not limited to publishing an erratum or corrigendum (correction); retracting the article (removing it from the journal); taking up the matter with the head of department or dean of the author’s institution and/or relevant academic bodies or societies; banning the author from publication in the HelJVES or all journals, or appropriate legal action.


