Early outcomes of fenestrated and/or branched endovascular repair of complex aortic aneurysms (F/B-EVAR). A single-center experience

| Available Online: | September, 2023 |
| Page: | 79-86 |
Author for correspondence:
Tzimkas-Dakis Konstantinos
Vascular Surgery Trainee, University of Thessaly, Mezourlo, 41110, Larissa, Greece
Τel: +302413501739
E-mail: kostasdakis1994@gmail.com
doi: 10.59037/73xm1062
ISSN 2732-7175 / 2023 Hellenic Society of Vascular and Endovascular Surgery Published by Rotonda Publications
All rights reserved. https://www.heljves.com
Abstract
Full Text
References
Image
Abstract
Abstract:
Introduction: Open surgical repair of juxta-, para- and thoracoabdominal aortic aneurysms (TAAA) has been the gold-standard for patients fit for surgery. However, endovascular repair of complex aortic aneurysms using devices im- plementing directional branches or fenestrations for incorporation of reno-visceral target vessels (TV) has gained wide- spread attention, due to its lower mortality and complication rate and its more attractive profile for high-risk patients. Nonetheless, complex endovascular technologies require meticulous abidance to protocols for successful application.
Methods: A retrospective analysis of prospectively collected data from a single, tertiary center was undertaken, includ- ing all patients undergoing complex aortic endovascular repair with fenestrated (F-EVAR) of branched devices (B-EVAR) from a 5-year time period (2018-2023). Primary outcomes included 30-day mortality, while secondary outcomes includ- ed primary TV patency, acute kidney injury (AKI), spinal cord ischemia (SCI), myocardial infarction (MI) and stroke rates.
Results: Of 74 patients (mean age: 69 ± 5.6 years-old, 98% males), 31 (42.6) and 43 (57.6%) were treated by F-EVAR and B-EVAR, respectively. Mean aneurysm diameter was 68 ± 1.8cm, with 15 (20.5%) juxtarenal AAA, 27 (35.6%) pararenal AAA, 16 (21.9%) type IV TAAA, 6 (8.2%) type III TAAA, and 10 (13.7%) type II TAAA treated. Sixteen (21.6%) patients were treated due to failed-EVAR. In total, 272 TV were successfully revascularized. Thirty-day mortality was 8.1%. Primary TV patency rate was 99.2% (270/272). Endoleak rate was 8.1% (6/74). No cases of AKI or MI were observed. One (1.3%) case of hemorrhagic stroke was observed. Three cases (4%) of SCI were observed, including one temporary paraparesis and two permanent paraplegia cases. Reintervention rates was 5.4%, including two cases of renal artery stent thrombosis revascularization procedures.
Conclusions: Endovascular repair of complex aortic aneurysms is feasible, with good perioperative outcomes. Adher- ence to precise protocols covering every technical aspect of patient care is warranted for satisfactory outcomes.
Keywords: B-EVAR, F-EVAR, pararenal, juxtarenal, thoracoabdominal
Full Text
INTRODUCTION
Juxtarenal and pararenal abdominal aortic aneurysm represent approximately 15% of all abdominal aortic aneurysms, while thoracoabdominal aortic aneurysms (TAAA) have a reported prevalence of 5.9/100.000 person years.1,2 Historically, open surgical repair has been considered the standard treatment for complex aortic aneurysms and it was recommended in the guidelines for patients fit to undergo conventional repair.3,4 However, open surgical repair has been traditionally associated with extensive thoracoabdominal exposure, proximal aortic cross-clamping, prolonged visceral and renal ischemia, high cardiovascular, pulmonary and renal morbidity rates, as well as increased intensive care unit (ICU) and hospital stay.5-7 Endovascular strategies seem to be superior to open surgical repair in terms of early morbidity and mortality, gradually evolving as the first-line treatment for complex aortic aneurysm of the descending thoracic and abdominal aorta.
Incorporation of renal and visceral vessels using fenestrated or branched endovascular repair (F/B-EVAR) have gained widespread use over the last decade, allowing for suprarenal and/or supravisceral aneurysm exclusion.8,9 These modalities have been associated with lower early perioperative mortality and morbidity rates, in addition to lower rates of acute kidney injury and spinal cord ischemia in the immediate postoperative period, when compared to traditional open surgical repair patient cohorts.10,11 Target vessel bridging stent-graft patency rates appear high in the short-term follow-up, gradually decreasing during mid-term to long-term postoperative surveillance, a phenomenon especially observed in renal artery stent-grafts.12,13
Robust data indicate that concentrating perioperative expertise in addition to centralizing services is crucial for high-quality aortic care. High-volume aortic centers have been shown to provide state-of-the-art care for patients with complex aortic lesions, improving their outcomes with time.14,15
In the current analysis, we aim to present the 30-day outcomes following endovascular repair of complex aortic aneurysms in a single-tertiary center during a 5-year period.
METHODS
A single-center, observational retrospective study of prospectively collected data was conducted. All patients who underwent endovascular repair of complex aortic aneurysms during a 5-year period (May 2018 – May 2023) in a vascular surgery department of a tertiary university hospital were included in the analysis. Patients that were treated with chimney EVAR (ChEVAR) technique were excluded. All included patients provided written informed consent. Data were collected prospectively and analyzed retrospectively by two (G.K., K.S.) experienced vascular surgeons who were involved in patients’ management and care. Demographic data (sex, age, comorbidities including hypertension (HTN), dyslipidemia (DL), coronary artery disease (CAD) diabetes mellitus (DM), chronic-obstructive pulmonary disease (COPD), history of smoking, atrial fibrillation (AF)) were collected.
Aneurysm-related definitions & Operative characteristics
Aneurysm-related characteristics, including maximum diameter, aneurysm type [pararenal, juxtarenal, suprarenal, thoracoabdominal type I-V (Safi modification of Crawford classification)16], clinical status (asymptomatic, symptomatic, rupture), prior standard endovascular aortic aneurysm repair (EVAR), prior presence and type of endoleak were also collected. Aneurysms with an aortic neck <5mm from the lowest renal artery were defined as juxtarenal. Aneurysms in which at least one renal artery originated from the aneurysm sac were defined as pararenal and aneurysms involving the origin of one or more visceral (celiac artery, superior mesenteric artery) without extending above the diaphragm were defined as suprarenal, partially following reporting the suggested terminology of the reported standards on complex aortic repair.17 Operative details collected included the type of endovascular repair (F-EVAR, B-EVAR, combination of both), type of aortic endograft deployed, number of target vessels (TV) incorporated and type of bridging stent-grafts deployed.
Outcomes – Definitions
Outcomes during the initial 30-day postoperative period were assessed. Primary outcomes included primary technical success, defined as successful introduction and deployment of the device in the absence of surgical conversion or mortality, type I or type III endoleak, branch occlusion, or graft limb obstruction.17 Secondary outcomes included target vessel (TV) primary patency, presence and type of endoleak in follow up CTA, reintervention, acute kidney injury, spinal cord ischemia, stroke (ischemic, hemorrhagic) and myocardial infarction (MI). Definitions and reporting of outcomes regarding primary technical success, acute kidney injury (AKI), myocardial infarction (MI), presence and type of endoleaks, reintervention, and major cerebral events/stroke were carried out based on the Reporting Standards for endovascular aortic repair of aneurysm involving the renal-mesenteric arteries.17
Initial Evaluation
All patients presenting in the outpatient clinic or the emergency department with a complex aortic aneurysm who were treated by F/B-EVAR were included. All patients received a complete clinical assessment by a vascular surgeon, underwent a complete blood work-up and computed tomography angiography (CTA) of the aorta and the iliac arteries. Imaging studies were assessed in a dedicated evaluation software (3mension Vascular, Pie Medical Imaging, Philipsweg 1 6227 AJ, Maastricht, The Netherlands) and the endovascular operative strategy was evaluated and decided by two of four vascular surgeons with experience in complex aortic repair (M.M., A.G., G.K. K.S.). Type of endovascular repair was decided following measurement, planning, and sizing. Adherence to Instructions for Use (IFU) was absolute in all cases where “off-the-shelf” devices were utilized.
Operative Details
Techniques for vascular access, device orientation and implementation, as well as target vessel incorporation and catheterization have already been described.18 Intravenous unfractioned heparin is administered (5000 IU) for an ACT of >250sec, measured every 30 minutes, with additional heparin infusions for target ACT maintenance. Patent left subclavian artery, as well as hypogastric arteries, is considered the standard of care, as we opt for their patency conservation of them, aiming towards stroke and spinal cord ischemia protection. During the last year, we opted for an increased number of two-staged procedures, especially regarding elective B-EVAR procedures with increased aortic coverage, not implementing a directional side branch for spinal cord blood supply conditioning. Regarding the fenestrated devices, all were provided by a single platform (COOK Medical, Bloomington, Indiana, USA). Regarding branched endovascular repair, devices were provided by different platforms (COOK Medical, Bloomington, Indiana, USA and ARTIVION, 1655 Roberts, Kennesaw, USA) and incorporated either outer or inner directional branches for visceral vessels. Upper extremity vascular access (axillary artery) was utilized in all B-EVAR cases, while it was used in specific anatomies or as a bail-out option in F-EVAR cases. Deployed bridging stentgrafts for visceral vessel incorporation included both balloon-expandable (BXCS) and self-expanding (SXCS) covered stentgrafts, while relining was not routine and was opted for per surgeon preference.
Postoperative Surveillance
Our aim was to extubate every patient in the operation theatre after the end of the operation and thus not admit them to the ICU. Following a 2-hour surveillance period in the resuscitation suite, unless an extended close-up surveillance was needed, all patients were transferred to the Vascular Surgery ward for postoperative monitoring. Our strategy according to antiplatelet therapy is the administration of aspirin alone (acetylsalicylic acid 100mg od) preoperatively as well as in the immediate first 24hour period, allowing for spinal cord drainage in cases of acute SCI. Dual antiplatelet medication is administered after the 1st postoperative day (adding clopidogrel 75mg od), following a complete motor and sensory evaluation of the patient. For the initial 24-hour period, all patients were under close monitoring, including arterial blood pressure, ECG and hourly urine output evaluation. All patients are mobilized during the 1st postoperative day and were started on a clear-liquid diet, unless contraindicated. Following discharge, all patients undergo a complete clinical evaluation and blood-work up at the postoperative 30-day mark and re-evaluated at the outpatient clinic. CTA of the aorta and the iliac arteries was undertaken either during the hospital stay of the patient (followed by an ultrasound evaluation during the 30-day mark) or at the 30-day mark, at the discretion of the operating physician. Patients are evaluated every 6 months during the 1st postoperative year by clinical evaluation, blood workup and via triplex ultrasound, and yearly afterwards. CTA is performed at the 1 postoperative year hallmark and every year afterwards. Should any concerns arise (sac expansion, evidence of endoleak or target vessel occlusion) a new CTA is performed for further diagnostic follow-up.
RESULTS
From May 2018 to May 2023, a total of 74 patients (mean age: 69 ± 5.6 years-old, 98% males) were treated with a F/B-EVAR device. Patients’ comorbidities are shown on Table 1. Most patients (n=60, 81%) were treated electively (asymptomatic), while 11 (14.8%) patients were treated for a symptomatic presentation of an intact aneurysm, one patient was treated for an aortoenteric fistula following open surgical repair (1.3%) and two (2.7%) patients presented with a ruptured aneurysm. Sixteen (21.6%) were treated on the grounds of a prior failed-EVAR, 81.2% (13/16) of whom presented with a type Ia endoleak.
Aneurysm-Related & Technical Characteristics
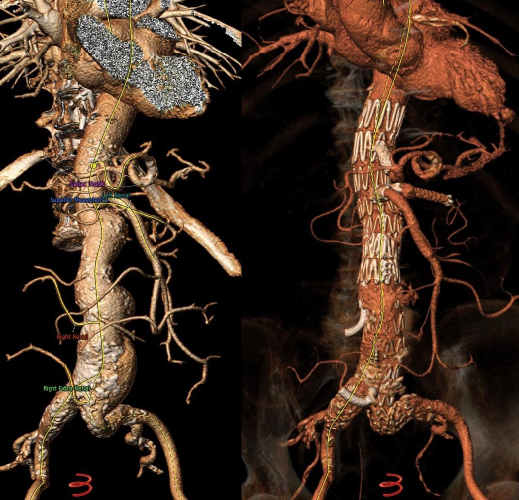
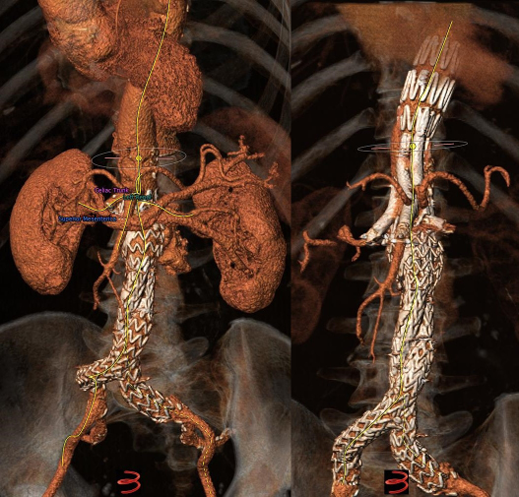
Mean aneurysm diameter was 68 ± 1.8cm. Type of aortic aneurysms treated included 15 (20.2%) juxtarenal AAA, 27 (36.4%) pararenal AAA, 16 (21.6%) type IV TAAA, 6 (8.1%) type III TAAA, and 10 (13.5%) type II TAAA. Regarding the type of endograft, 30 (40.5%) were fenestrated and 43 (58%) were branched devices, while one patient (1.5%) was treated with a combination of a fenestrated and a branched device. Regarding fenestrated devices, all were provided by COOK (COOK Medical, Bloomington, Indiana, USA), and included 1 to 4 fenestrations based on patient anatomy. Regarding branched endovascular repair, 31 (41.9%) of patients were treated with an “off-the-shelf” outer branched device (T-Branch Platform, COOK Medical, Bloomington, Indiana, USA) (Figure 1) and 4 (5.4%) patients were treated with an “off-the-shelf” inner branched device (E-NSIDE Multibranch Stentgraft System, ARTIVION, 1655 Roberts, Kennesaw, USA). Additionally, eight (10.8%) patients were treated with a custom-made branched device, including six outer-branched and two inner branched devices. Five custom-made devices (all outer-branched) were provided by COOK (COOK Medical, Bloomington, Indiana, USA), while three custom-made devices (two inner-branched, one outer-branched) were provided by ARTIVION (ARTIVION, 1655 Roberts, Kennesaw, USA). A combination of a fenestrated and a custom-made branched device for the implementation of 5 target vessels (one ancillary right renal artery) was used in one case (Figure 2), as well as a custom-made 5-branced device was used (separate common hepatic and splenic artery) in a failed EVAR case (Figure 3).
Target Vessels
A total of 272 target vessels (TVs) were incorporated during F/B-EVAR, including 68 (25%) right renal arteries (RRA), 72 (26.4%) left renal arteries (LRA), 65 (23.9%) celiac arteries (CA) and 67 (24.6%) superior mesenteric arteries (SMA), for a mean 3.67 vessels/patient. Regarding bridging stentgrafts, 219 BeGraft Peripheral balloon-expandable covered stentgrafts (Bentley InnoMed GmbH, Hechigen, Germany), 48 VBX balloon expandable covered stentgrafts (GORE Medical, W.L.Gore & Associates Inc., Flagstaff Arizona, USA), 1 Advanta balloon expandable covered stentgrafts (Getinge, Goteborg, Sweden), 2 VIABAHN self-expandable covered stentgrafts (GORE Medical, W.L.Gore & Associates Inc., Flagstaff Arizona, USA) and 2 Fluency balloon expandable covered stentgraft (BD Switzerland Eysins, Vaud. Switzerland) were deployed for target vessel implementation. Self-expanding or balloon-expandable bare metal stentgrafts were deployed for relining per surgeon preference in 22 target vessels. In total, there were two RRA and one LRA with preoperative occlusion either due to atherosclerotic disease or due to prior EVAR complications. Two patients had an ancillary RRA, one patient had an ancillary LRA and one patient had a separate orifice of the common hepatic and splenic artery. Additionally, two patients had ectopic RRA, while one patient had a prior revascularized LRA via the periscope technique. All the above-mentioned TV were successfully catheterized and stented. In four patients the celiac artery was left unstented for spinal cord conditioning to reduce the risk of spinal cord ischemia, as the patients were deemed high risk, and was subsequently stented in a 2nd stage operation, without spinal cord ischemia. In one case of F-EVAR, failure to stent the LRA via the femoral approach was treated in a 2nd stage operation with successful catheterization and stenting through upper extremity access. Type of bridging stentgraft per target vessel, as well as relining are shown on Table 2.
Primary & Secondary Outcomes
Primary technical success was 98.6% (73/74) as defined in the reporting standards for endovascular repair of aneurysms involving the renal-mesenteric arteries.17 In one patient left renal artery catheterization and stenting was not achieved during the initial procedure, and a reintervention was rescheduled, which was successful. No surgical conversion or mortality, type I or type III endoleak, branch occlusion, or graft limb obstruction was observed in all 74 patients.
30-days outcomes – Mortality
Following F/B-EVAR, 30-day mortality was 8.1% (6/74). One patient with severe coronary artery disease and a type II TAAA died in the first postoperative day following cardiac arrest immediately after B-EVAR and carotid-subclavian bypass. One patient died in the first postoperative day due to external iliac artery rupture and uncontrolled hemorrhage. One patient with history of mechanical mitral valve repair, priorly under treatment with acenocoumarol, died in the 7th postoperative day following massive cerebral hemorrhage. Two patients died following ICU admission due to multiorgan failure. Reasons for ICU admission included one patient with pulmonary complications with COPD history and SCI following B-EVAR and one patient with aortoenteric fisula with primary bowel repair following B-EVAR. Finally, one patient died following COVID-19 related sepsis and pulmonary complications.
30-day outcomes – Target Vessel Patency/Complications/Reinterventions
Primary TV patency at 30-days was 99.2% (270/272), with cases of left renal artery branch thrombosis, which were successfully revascularized. No cases of AKI or myocardial infarction were observed. One case of stroke (1.3%) (hemorrhagic) as mentioned before was observed. Three cases (4.1%) of spinal cord ischemia were observed, including one case of temporary paraparesis with ipsilateral motor and sensory loss (Grade 2) and two cases of permanent paraplegia with complete motor and sensory loss, as well as urinary and anal sphincter insufficiency (Grade 3c). In all cases, clinical image was correlated with spinal cord ischemia findings confirmed by spinal cord magnetic resolution imaging as well as neurologic and neurosurgical evaluation. Endoleak rate was 8.1% (6/74), with two type II, two type IIIa and two IIIc endoleaks. All type II and IIIc endoleaks self-resolved and did not lead to sac expansion, as confirmed by the postoperative 30-day CTA scan, and one case was treated with endograft relining, leading to complete resolve of the endoleak. Reintervention was required in a total of 4 cases (5.4%). In two cases, TV occlusion (both regarding a renal artery branch) due to stent thrombosis was treated with thrombus aspiration and relining, without postoperative AKI. One patient presented with lower limb malperfusion due to common femoral artery dissection at the 1st postoperative day, which was repaired via direct dissection flap suture fixation. Two patients developed ipsilateral lower limb reperfusion syndrome due to extended device sheath placement in the common femoral artery, with one case requiring major lower limb transfemoral amputation due to inadvertent ischemia.
DISCUSSION
Since their introduction over 20 years ago as treatment options for repair of complex aortic pathologies of the thoraco-abdominal aorta, fenestrated and branched endovascular repair have been traditionally offered to patients deemed unfit for conventional open surgical repair.19,20 Guidelines recommend open surgical repair as the treatment modality of choice for complex aortic lesions in fit patients.3,4 However, data over the last decade highlight a shift towards endovascular strategies as the first-line treatment option.8,21 In this study, we report the early experience of a single tertiary center, treating patients with complex aortic aneurysm by endovascular means.
In this study, technical success was high (>98%), conforming with international standards of high-volume centers. Reports suggests primary technical success rates over 95%, even in exceptionally complex cases, including endovascular repair following previous “failed” EVAR as well as post-dissection aortic degeneration.
In the current analysis, we present an 8.2% 30-day mortality rate, which falls within reported rates of perioperative mortality ranging between 2.6% and 8.6% of similar cohorts in the literature.22,23 Recent analyses on open conventional repair of thoracoabdominal AAA report mortality rates as high as 14.7%. Excluding treatment of elective, intact TAAA, open surgical repair of TAAA in cases of emergency, including both symptomatic and ruptured cases, has been associated with mortality rates ranging from 30% to 50%.24,25 On the contrary, large patient cohorts from high volume centers suggest low perioperative mortality rates following complex endovascular aortic repair, with a 5-fold decrease in mortality when compared to open repair.21,26
Target vessel (TV) revascularization has continued to be optimized since the first implementation of fenestrated and branched devices, focusing on lowering patency related complications.12 High-volume centers reported target patency rates of over 98% during the 30-day postoperative period27,28, while data on 1-year patency rates range from 90% to 96.2%.29,30 Renal arteries are associated with a higher risk of stent instability following implementation during F/B-EVAR.12. In our cohort, primary technical success for TV revascularization was 99.2%. Two cases of renal artery stent thrombosis were successfully revascularized, preventing end-organ failure.
Besides endoleaks observed in standard EVAR for infrarenal AAA, target vessel related endoleaks have been reported in F/B-EVAR. Numerous factors, including target vessel tortuosity, number of bridging stentgrafts deployed, type of target vessel and relining have been associated with increased TV endoleak rates. Two cases of type IIIc endoleaks were observed in our cohort, both of which resolved spontaneously, confirmed by postoperative CTA scans during 30-day follow-up. Literature suggests that, especially in B-EVAR, such endoleaks (attachment aortic side-branch or side branch-side branch endoleaks) are benign and generally do not require reintervention in the majority of cases.31 Especially intra-operative type IIIc endoleaks, as high doses of intravenous unfractioned heparin have been already administered to maintain an ACT value of >250 seconds.
Major stroke, myocardial infarction, acute kidney injury and spinal cord ischemia all attribute to the perioperative morbidity profile of TAAA repair. Considering the incorporation of renal arteries in complex endovascular repair, either by fenestrations or directional branches, as well as the use of intravenous contrast material during perioperatively, acute kidney injury is a common complication. Our experience, although relatively confined, shows zero rates of perioperative AKI and no case of postoperative need for temporary or permanent dialysis. Older reports suggest AKI rates as high as 40%, while more recent studies suggest lower rates of both AKI (~14%) as well as postoperative need for permanent dialysis.22,32,33 Long-term follow-up of patients is warranted as they are in high risk for chronic kidney disease, suggested by reports on decrease in renal function up to 35% in some series.34
Regarding major cerebral events, either ischemic or hemorrhagic, rates following F/B-EVAR are reported low, approximately <1%.35 In our series, only one case of postoperative hemorrhagic stroke was reported (1.5%). Upper extremity access for branched or fenestrated endovascular repair has been associated with increased stroke rates, with recent analyses do not support that right-sided upper access is always associated with higher cerebral event rate. In our case, the patient had undergone a carotid-subclavian bypass as a 1st stage repair for a type II TAAA and had a mechanical mitral valve replacement 5-years prior, two important risk factors.
Spinal cord ischemia presenting with either temporary or permanent paraparesis or paraplegia is another dreadful complication which ought to be expected in all patients undergoing complex endovascular aortic repair. Reports in the literature indicate that paraparesis and paraplegia most frequently occur during the first 24-hour period, with rates ranging from 4% to 31% for total spinal cord ischemia. A number of factors has been associated with SCI, including preoperative renal impairment, extended aortic coverage, operation times, clinical presentation of the aneurysm among other.11 In our cohort, we consider a SCI rate of 4.1% considerable. In all three cases of both temporary and permanent SCI we identified known risk factors, as all the patients had undergone extensive aortic coverage, had lower preoperative glomerular filtration rate of <60mL/min/1.73m2, while two were symptomatic and one was a rupture case. Nevertheless, all departments performing either open or endovascular repair of complex aortic aneurysms should follow strict protocols in cases of SCI. Two staged procedures, mainly in elective cases, have been shown to induce spinal cord blood supply conditioning. During the last year, we have opted for at least 5 cases of two-staged procedures, especially in cases with extensive aortic coverage, aiming towards the reduction of spinal cord ischemia risk. Reports suggest both lower rates as well as limited severity of neurologic deficits associated with spinal cord ischemia in patients undergoing two-staged procedures.36-38 In our department, two-staged procedures allowing for temporary aneurysm sac perfusion via one directional side-branch are selected in patients with anatomic criteria characterized as high-risk for SCI (“shaggy” aorta, large intercostal arteries, type II TAAA, occluded hypogastric arteries).
Limitations
Our analysis is associated with a number of limitations, partially confining the generated outcomes. Firstly, the retrospectively analyzed data, regardless of the prospective nature of their collection, bears a certain degree of bias. Moreover, the relatively low number of cases and major adverse events limit the objectiveness of the reported outcomes. Additionally, some important preoperative and operational parameters, including blood loss, need for blood product transfusion, and radiation exposure were not systematically recorded, while their analysis would provide further information regarding holistic patient evaluation and possible association with the reported outcomes.
CONCLUSIONS
Complex aortic repair using fenestrated or branched devices is feasible and can be achieved with good perioperative outcomes. Vascular centers performing complex endovascular aortic procedures should have specific preoperative, intraoperative, postoperative, as well as long-term follow-up protocols, aiming towards the early diagnosis and reintervention in cases of complications.
References
- Jongkind V, Akkersdijk GJM, Yeung ΚΚ, Wisselink W. A systematic review of endovascular treatment of extensive aortoiliac occlusive disease. J Vasc Surg. 2010;52(5):1376-83.
- Blake-Guirguis JM, Beil TL, Senger CA, Coppola EL. Primary Care Screening for Abdominal Aortic Aneurysm: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2019;322(22):2219-2238.
- Isselbacher EM, Preventza O, Black Lii JH, Augoustides JG, Beck AW, Bolen MA, et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 13;80(24):e223-e393.
- Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Conhert T, et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg. 2019;57(1):8-93.
- Huynh TTt, Miller 3rd CC, Estrera AL, Sheinbaum R, Allen SJ, Safi HJ. Determinants of hospital length of stay after thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2002;35(4):648-53.
- Conrad MF, Crawford RS, Davison JK, Cambria RP. Thoracoabdominal aneurysm repair: a 20-year perspective. Ann Thorac Surg. 2007;83(2):S856-61; discussion S890-2.
- Coselli JS, LeMaire SA, Preventza O, De la Cruz KI, Cooley DA, Price MD, et al. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg. 2016;151(5):1323-37.
- O’Donnell TFX, Patel VI, Deery SE, Li C, Swerdlow NJ, Liang P, et al. The state of complex endovascular abdominal aortic aneurysm repairs in the Vascular Quality Initiative. J Vasc Surg. 2019;70(2):369-380.
- Suckow BD, Goodney PP, Columbo JA, Kang R, Stone DH, Sedrakyan A, et al. National trends in open surgical, endovascular, and branched-fenestrated endovascular aortic aneurysm repair in Medicare patients. J Vasc Surg. 2018;67(6):1690-1697.e1.
- Lommen MJ, Vogel JJ, VandenHull A, Reed V, Pohlson K, Answini GA, et al. Incidence of Acute and Chronic Renal Failure Following Branched Endovascular Repair of Complex Aortic Aneurysms. Ann Vasc Surg. 2021; 76: 232-243.
- Spanos K, Kölbel T, Kubitz JC, Wipper S, Konstantinou N, Heidemann F, et al. Risk of spinal cord ischemia after fenestrated or branched endovascular repair of complex aortic aneurysms. J Vasc Surg. 2019;69(2):357-366.
- Resch T, de Vries J-P, Haulon S. Optimising Target Vessel Patency after Complex Aortic Repair: Things We Know that We Know. Eur J Vasc Endovasc Surg. 2021;62(1):4-6.
- Spanos K, Jakimowicz T, Nana P, Behrendt CA, Panuccio G, Kouvelos G, et al. Outcomes of Directional Branches of the T-Branch Off-the-Shelf Multi-Branched Stent-Graft. J Clin Med. 2022;11(21): 6513
- Karthikesalingam A, Hinchliffe RJ, Loftus IM, Thompson MM, Holt PJ. Volume-outcome relationships in vascular surgery: the current status. J Endovasc Ther. 2010;17(3):356-65.
- Reames BN, Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and operative mortality in the modern era. Ann Surg. 2014;260(2):244-51.
- Crawford ES, Coselli JS. Thoracoabdominal aneurysm surgery. Semin Thorac Cardiovasc Surg 1991;3:300-22.
- Oderich GS, Forbes TL, Chaer R, Davies MG, Lindsay TF, Mastracci T, et al. Reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries. J Vasc Surg. 2021;73(1S):4S-52S.
- Chait J, Mendes BC, DeMartino RR. Anatomic factors to guide patient selection for fenestrated-branched endovascular aortic repair. Sem Vasc Surg. 2022; 35(3): 259-2709.
- Chuter TA, Gordon RL, Reilly LM, Goodman JD, Messina LM. An endovascular system for thoracoabdominal aortic aneurysm repair. J Endovasc Ther. 2001;8(1):25-33.
- Mastracci TM, Greenberg RK, Hernandez AV, Morales C. Defining high risk in endovascular aneurysm repair. J Vasc Surg. 2010;51(5):1088-1095.e1.
- Ultee KH, Zettervall SL, Soden PA, Darling J, Verhagen HJM, Schermerhorn ML. Perioperative outcome of endovascular repair for complex abdominal aortic aneurysms. J Vasc Surg. 2017;65(6):1567-1575.
- Tran K, Lee AM, McFarland GE, Sgroi MD, Lee JT. Complex endovascular aneurysm repair is associated with higher perioperative mortality but not late mortality compared with infrarenal endovascular aneurysm repair among octogenarians. J Vasc Surg. 2019;69(2):327-333.
- Nordon IM, Hinchliffe RJ, Holt PJ, Loftus IM. Modern treatment of juxtarenal abdominal aortic aneurysms with fenestrated endografting and open repair–a systematic review. Eur J Vasc Endovasc Surg. 2009;38(1):35-41.
- Latz CA, Boitano L, Schwartz S, Swerdlow N, Dansey K, Varkevisser RRB, et al. Contemporary mortality after emergent open repair of complex abdominal aortic aneurysms. J Vasc Surg. 2021;73(1):39-47.e1.
- Latz CA, Boitano L, Schwartz S, Swedlown N, Dansey K, Varkevisser RRB, et al. Editor’s Choice – Mortality is High Following Elective Open Repair of Complex Abdominal Aortic Aneurysms. Eur J Vasc Endovasc Surg. 2021;61(1):90-97.
- Varkevisser RRB, O’Donnell TFX, Swerdlow NJ, Liang P, Chun L, Ultee KH, et al. Fenestrated endovascular aneurysm repair is associated with lower perioperative morbidity and mortality compared with open repair for complex abdominal aortic aneurysms. J Vasc Surg. 2019;69(6):1670-1678.
- Mastracci TM, Greenberg RK, Eagleton MJ, Hernandez AV. Durability of branches in branched and fenestrated endografts. J Vasc Surg. 2013;57(4):926-33; discussion 933.
- Abdelhalim MA, Tenorio ER, Oderich GS, Haulon S, Warren G, Adam D, et al. Multicenter trans-Atlantic experience with fenestrated-branched endovascular aortic repair of chronic post-dissection thoracoabdominal aortic aneurysms. J Vasc Surg. 2023;78(4):854-862.e1.
- Van Calster K, Bianchini A, Elias F, Hertault A, Azzaoui R, Fabre D, et al. Risk factors for early and late mortality after fenestrated and branched endovascular repair of complex aneurysms. J Vasc Surg. 2019;69(5):1342-1355.
- Oderich GS, Greenberg RK, Farber M, Lyden S, Sanchez L, Fairman R, et al. Results of the United States multicenter prospective study evaluating the Zenith fenestrated endovascular graft for treatment of juxtarenal abdominal aortic aneurysms. J Vasc Surg. 2014;60(6):1420-8.e1-5.
- Kärkkäinen JM, Tenorio ER, Jain A, Mendes BC, Macedo TA, Pather K, et al. Outcomes of target vessel endoleaks after fenestrated-branched endovascular aortic repair. J Vasc Surg. 2020;72(2):445-455.
- Haddad F, Greenberg RK, Walker E, Nally J, O’Neill S, Kolin G, et al. Fenestrated endovascular grafting: The renal side of the story. J Vasc Surg. 2005;41(2):181-90.
- Marzelle J, Presles E, Becquemin JP, WINDOWS Trial Participants. Results and factors affecting early outcome of fenestrated and/or branched stent grafts for aortic aneurysms: a multicenter prospective study. Ann Surg. 2015;261(1):197-206.
- Cucuruz B, Kasprzak PM, Gallis K, Schierling W, Pfister K, Kopp R. Midterm outcome of renal function after branched thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2020;71(4):1119-1127.
- Swerdlow NJ, Liang P, Li C, Dansey K, O’Donnell TFX, de Guerre LEVM, et al. Stroke rate after endovascular aortic interventions in the Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2020;72(5):1593-1601.
- O’Callaghan A, Mastracci TA, Eagleton MJ. Staged endovascular repair of thoracoabdominal aortic aneurysms limits incidence and severity of spinal cord ischemia. J Vasc Surg. 2015;61(2):347-354.e1.
- Harrison SC, Agu O, Harris PL, Ivancev K. Elective sac perfusion to reduce the risk of neurologic events following endovascular repair of thoracoabdominal aneurysms. J Vasc Surg. 2012;55(4):1202-5.
- Kasprzak PM, Gallis K, Cucuruz B, Pfister K, Janotta M, Kopp R. Editor’s choice–Temporary aneurysm sac perfusion as an adjunct for prevention of spinal cord ischemia after branched endovascular repair of thoracoabdominal aneurysms. Eur J Vasc Endovasc Surg. 2014;48(3):258-65.