Pros and Cons of Contrast-Enhanced Ultrasound in Endovascular Aneurysm Repair Surveillance

| Available Online: | September, 2023 |
| Page: | 89-98 |
Author for correspondence:
Emmanouil Barmparessos, MD MSc
St George’s Vascular Institute, St George’s University Hospital, Blackshaw Road, London, SW17 0QT, UK
Τel: +44 7999240364
E-mail: ebarmpar@sgul.ac.uk
doi: 10.59037/gbgm4v65
ISSN 2732-7175 / 2023 Hellenic Society of Vascular and Endovascular Surgery Published by Rotonda Publications
All rights reserved. https://www.heljves.com
Abstract
Full Text
References
Image
Abstract
Abstract:
Endovascular Aneurysm Repair (EVAR) has become an increasingly popular treatment for abdominal aortic aneurysm. The success of this type of treatment is dependent upon, to great extent, appropriate post-procedure surveillance that accurately recognises complications in a timely fashion. The prominent modes of surveillance have been Computed Tomography Angiography (CTA) and Color Duplex Ultrasound (CDUS). Each imaging method has its limitations; particularly the use of radiation and intravenous contrast media for the CTA and the subjectivity and lower resolution of the images that CDUS generates. More recently, Contrast-Enhanced Ultrasound (CEUS) has been used and provides an accurate diagnosis of many post-procedure complications. This study aims to review the literature and present the evidence for the role of CEUS in the surveillance of EVAR patients, which may have implications for future practice.
Keywords:Contrast, Ultrasound, Aneurysm, Aorta, Endovascular repair, Surveillance
Full Text
INTRODUCTION
Endovascular aneurysm repair (EVAR) has become the dominant treatment in many centres for abdominal aortic aneurysm (AAA).1 However, the ideal strategy for surveillance of patients following EVAR is still under debate.2 Due to the need for life-long surveillance, the potential consequences of the regular imaging examination on patients’ health and healthcare logistics cannot be ignored.3 Contrast-Enhanced Ultrasound scan (CEUS) has been considered as an imaging modality for EVAR patient surveillance as it can offer safe and accurate surveillance without significant safety limitations. This study aims to review the salient literature and investigate the technique of CEUS with its pros and cons in an EVAR Surveillance programme.
EVAR Surveillance
Despite the very good short- and mid-term outcomes of EVAR, data suggest a high risk of reintervention in the long term due to complications that may arise in the postoperative period (Table 1).4 The recently published data of the 15-year follow-up of EVAR-1 trial patients highlighted the importance of surveillance after the index procedure.5
Various imaging modalities have been used for EVAR surveillance (Table 2). Computed Tomography Angiography (CTA) and Color Doppler Ultra Sound (CDUS) are the most common methods of EVAR surveillance. Due to the absence of conclusive data regarding the optimum surveillance regimen, variation exists between institutions.2,10 Considering the increasing life expectancy and the need for life-long surveillance, the need for a carefully configured surveillance programme is imperative.11
The aims and the characteristics of the optimal EVAR surveillance programme are summarized in Table 2. The Society of Vascular Surgery (SVS) and European Society of Vascular Surgery (ESVS) suggested recently that EVAR patients should be stratified during surveillance to high, moderate and low risk for complications, based on the adequacy of endograft sealing, the stability of the aneurysm diameter and the absence of high flow endoleak. Following this stratification, the intensity of monitoring could be tailored to the individual patient, using either CTA or annual CDUS.6,12
Various reports have tried to clarify the role of contrast ultrasonography in EVAR surveillance programs.13-16 The role of contrast ultrasonography could be adjunctive in cases where the CTA is non-diagnostic as a problem solving tool, or it could replace the annual CTA scans of EVAR patients in order to decrease the use of iodine-based contrast and ionizing radiation. Finally, it could be the first-line method for a patient with poor renal function.17
Ultrasound contrast agents
Ultrasound contrast agents (UCA) consist of microbubbles. Gas is enveloped by a phospholipidic or albumin cap creating the microbubbles. The ultrasound waves cause the resonation of the microbubbles which backscatter in an enhanced and non-linear way into subharmonic and superharmonic signals. Conversely, surrounding parenchymal tissue follows linear scattering.18 The higher the insonation power [Mechanical Index(MI)] used, the greater the oscillation of the microbubbles that is produced. Persistent oscillation of the microbubbles, however, causes their rupture and disappearance.19 By using algorithms of pulse inversion, it is possible to create images with high contrast. The first generation of contrast constituted fragile microbubbles which were demolished briefly by the insonation power as CDUS was initially used with high MI. Due to the microbubble’s destruction, the high-intensity signals were created only intermittently.20 The second generation of UCA has a more flexible cap and can be utilized even with very limited MI, where the microbubbles remain intact. The most contemporary ultrasound devices can create sufficient images with less than 0.1 up to 0.05 MI, where the microbubbles retain their shape.
SonoVue (Bracco International B.V., Amsterdam, The Netherlands) is a second-generation UCA, available across Europe and the United Kingdom, having been assessed extensively in a range of vascular pathologies. SonoVue contains microbubbles made from phospholipids which encircle sulphur hexafluoride; an inert, harmless gas. The mean diameter of the microbubbles is 2.5μm, with 90% of them having a diameter of less than 8μm; smaller than the red blood cells and thus optimal for assessment of the microcirculation, without dispersing from the endovascular space. Following intravenous administration, microbubbles can be identified in the arterial circulation after 10 to 30 seconds. The blood enhancement persists for 2 to 5 minutes, depending on the MI which is used. If the enhancement is lost, a repeat dose of UCA may be administered. The gas is excreted through the lungs and the phospholipidic cap is metabolized through the liver.21,22
The safety of UCA has been reported in various studies.23 Data derived from 30222 cases of UCA administration reveal the incidence of life-threatening allergic reactions to be 0.02%.24 UCA is not contraindicated in patients with impaired renal function. Previous allergic reactions, pulmonary hypertension, uncontrolled arterial hypertension and pregnancy have been reported as contraindications for UCA administration.25
Despite the accumulated experience, the ideal dose and way of UCA administration are still under debate.26 Administrating a single dose or two separate doses and a dose of 2.4 ml instead of 1.2 ml may have a significant impact on the value of the examination. A higher dose of UCA would create a greater enhancement; however, it would cause higher attenuation as the reflection and absorption of the ultrasound waves would be higher. Hence, the penetration of the insonation power would be lower and the visualization of the deep tissue would not be possible. A higher dose of UCA would lead to a blooming effect, which would make it difficult to distinguish between the areas of higher and lower enhancement because of the signal overlap. Conversely, a lower dose of UCA would cause sufficient enhancement in the initial primary arterial phase but it would be followed by lower enhancement in the delayed phase.19 Considering that the longer exposure of the UCA to the insonation would cause more microbubbles to rupture makes the situation more complicated.18 Iezzi et al reported in a study of 84 patients that a dose of 2.4ml of UCA had greater sensitivity in detecting endoleaks.27 A dose of 2.4 ml is suggested for the Sonovue; however, the dose may be adjusted depending on the case, region of interest and ultrasound device.28
The technique of CEUS for EVAR Surveillance
The examination environment must be safe, comfortable and warm enough before undertaking a contrast-enhanced ultrasound scan for EVAR surveillance. An ultrasound device equipped with the appropriate algorithms for CEUS as well as the UCA and vascular access should be available. As per hospital or practice policy, informed consent should be obtained.29 Vascular access involves a peripheral venous catheter (18-21 gauge) in the upper limb. A smaller calibre catheter may cause the microbubbles to rupture. A three-way stopcock facilitates convenient UCA administration and subsequent flushing with saline to advance the contrast into systematic circulation.30
The patient is placed in a supine or lateral position with the head slightly elevated at 10 degrees. Fasting for 8 to 12 hours before the scan will reduce the bowel gas artefact. Conventional ultrasound scan with a convex transducer (1-5MHz) in B-mode precedes the UCA administration. Commencing from the epigastrium, the scan is undertaken in the transverse and longitudinal plane, where the abdominal aorta and origins of the visceral branches are identified. By moving caudally, the proximal and distal sealing zones are assessed, as well as the patency of the branches in the case of fenestrated or branched EVAR. The maximum AAA diameter is calculated in the anterior-posterior axis and colour doppler mode is utilized to ascertain the presence of blood flow within the aneurysm sac [endoleak (EL)]. Spectral doppler provides a graphical measurement of the blood flow throughout the endograft, facilitating the diagnosis of possible stenosis due to endograft migration, endograft kinking or thrombus formation.31 The conventional sonographic investigation must be thorough before UCA administration, as artefacts already present at the initial scan may be exacerbated by the contrast enhancement.32 Mirror image artefact and acoustic shadow due to the total reflection of ultrasound waves may be intensified due to the settings that have been adopted for CEUS. Echogenic areas such as a severely calcified plaque may be erroneously considered as an area enhanced by contrast agents if the B-mode scanning has not been initially performed.33 The Gain setting should be adjusted carefully during the initial scan since excessively high gain would create brighter images where the interrogated tissue and the contrast agents might be difficult to distinguish. Most synchronous ultrasound devices have developed algorithms and stored settings which simplify CEUS.19,34
Following the initial scan and being familiar with the patient’s anatomy, it is possible to split the image display on the ultrasound monitor where images can be simultaneously viewed to allow comparison of the findings before and after the administration of UCA.35 Once the examiner has set the probe in the right position, an assistant may proceed with the reconstitution and intravenous infusion of the dispersion. 2.4 ml of the dispersion is given, and the catheter is flushed with 5 ml of saline. Immediately afterwards the scan is undertaken and is generally completed within five minutes. As with CTA, the arterial phase corresponds to the first 10 to 40 seconds following the UCA administration and the delayed phase at 90 to 300 seconds. The use of a timer may aid the investigation. Should the scan not be sufficiently diagnostic, a further dose of UCA may be given; however, it is useful to “clean” the interrogated area by increasing the MI, causing the microbubbles to rupture. Therefore, the replenishment of the contrast will be precisely assessed. The scanning is performed in coronal or sagittal view, depending on which plane the optimal visualization was accomplished by the B-mode scanning. In cases of obesity, bowel gas or abdominal hernias, the lateral position with a sagittal view has been shown to provide the best views. Data regarding the timing of endograft and aneurysm sac enhancement are collected.36 Simultaneous enhancement represents antegrade EL (Type I and Type III EL) while delayed enhancement represents retrograde EL [Type II endoleak (T2EL)]. The delayed phase is assessed in an interrupted fashion, as continuous resonation of the microbubbles would result in their rupture. Should any kind of endoleak be revealed, the scan is repeated with a new dose of contrast.37 If EL is suspected, the area of interest is focused on the proximal and distal sealing zones, and in the regions of overlap between endograft components. The usual site of origin of the Inferior mesenteric, lumbar and accessory renal arteries is investigated for possible back bleeding. The presence of these branches may be already known from the previous CTA; however, the branches derived from the posterior aspect of the aorta are difficult to evaluate.38
The advantages of CEUS in EVAR surveillance.
CEUS’ popularity has grown in recent years, as it represents a high-resolution imaging modality in which characteristics of the vessel wall can accurately be defined, and the blood flow dynamics can be investigated with high sensitivity.13 CEUS examination may be performed promptly and can be easily adapted to everyday clinical practice. The mean time to undertake the examination is 60 minutes, including 20 minutes of post-procedure patient monitoring.39 By utilizing algorithms specially developed for the CEUS, it is possible to visualize the volume of the blood flow with high resolution, as well as end-organ perfusion with high sensitivity.40 CEUS reinforces the performance of CDUS by identifying and classifying the type of EL and analyzing the blood vessel patency. The sensitivity of CDUS in EL detection varies significantly from 42.9% to 62.5% in studies where CTA is considered the gold standard modality for EL detection.41,42 Comparing CDUS with CEUS in EL detection, the sensitivity of CDUS is only 34% while the sensitivity of CEUS is 81%.39 Multiple reports have highlighted the high sensitivity of CEUS in revealing EL in EVAR patients which can reach even 100% when it is measured against the two-phase CTA.43
CEUS has the advantage over other imaging modalities as the contrast used is not nephrotoxic (Table 3). It has been reported that the incidence of iodine-based contrast-induced nephropathy can reach 10% in patients undergoing CTA in an outpatient setting.44 UCA is notably safe and multiple administrations are possible as it is withdrawn from the systemic circulation in 5 minutes.22 The incidence of adverse reactions following administration of UCA. has been reported between 0.02% to 0.08% with the majority of them being mild.24,45 While the incidence of adverse reactions following administration of iodine-based contrast agents is 0.7% to 3%.33,46
By adopting CEUS in EVAR surveillance, the total exposure to ionizing radiation is considerably reduced.47 The dose of ionizing radiation to EVAR patients may reach the levels of 24.0 mSv during the first year and may reach 8.0 mSv over the next year of surveillance.48 Ionizing radiation has been classified in Group 1 for carcinogenicity with the relative risk being higher as the cumulative radiation exposure increase.49
Beyond safety, CEUS has improved the accuracy of endoleak detection in EVAR surveillance.50 Contrary to CTA which provides a snapshot of the aneurysmal disease, CEUS investigates the blood flow in real-time, giving invaluable information regarding the velocity and the direction of flow inside the aneurysm sac.42 T2EL which is the commonest complication following EVAR still creates controversy regarding management.51 Although the risk of rupture is less than 1%, the natural history of T2EL is difficult to define, leading to a lack of clear guidelines regarding treatment.52 CEUS may help to recognize the type II endoleaks which require treatment.53 T2EL is further classified as type IIa when a single vessel creates a pool of blood into the aneurysmal sac, through a retrograde flow and type IIb when there are two or more feeding vessels.54 The classification into subtypes aimed to distinguish the pathophysiology of sac growth on the grounds of endoleak and therefore guiding optimal management.55 Monastiriotis et al. followed 382 EVAR patients over a year using CDUS and reported that the natural history of T2EL depends on the type and number of the culprit aortic branches as well as on the size and shape of the endoleak cavity.56 These four factors shaped the observed waveform patterns on CDUS surveillance, which were correlated with the clinical outcomes. CEUS as a dynamic imaging examination investigates the kinetics of the blood flow through the feeding vessels and characterises the endoleak cavity with higher sensitivity than CDUS.50 Bargellini et al investigated 18 patients with suspected T2EL by using CEUS and obtained quantitative measurements.57 They measured the delay of endoleak detection (wash-in) and disappearance (wash-out) following the administration of UCA. T2EL with delayed wash-in (≤100sec) and wash-out time (≥520 seconds) were defined as hypodynamic. By using a multiple logistic regression model, the slow wash-out time was the only independent predictor of AAA volume increase (≥1 mL per month). Interestingly, hypodynamic ELs were significantly related to inability of CTA to depict these ELs. Although the size of the cohort was small, the study did suggest that cases of T2EL detected by CTA did not necessarily lead to aneurysm growth and conversely T2EL not detected by CTA may lead to sac expansion, due to different haemodynamics.57
Another way of quantitative EL assessment using CEUS is by interrogating the perfusion of EL by measuring the enhancement created inside the aneurysmal sac and drawing time-to-intensity curves. Hence, by computing the maximum enhancement with respect to time and the area of enhancement, the type of EL can be distinguished as either high-flow (requiring prompt reintervention) or to slow-flow (requiring further monitoring by sequential imaging).38
The EVAR endograft contains metallic elements which create artefact in CTA scans obscuring the identification of EL. On the other hand, CEUS is significantly less susceptible to such artefact. Although the metallic structures can create artefact even with DUS, the use of UCA along with low insonation power and pulse-inversion algorithm may minimize the effect. Of similar importance, CEUS is useful in the follow-up of EVAR patients who have been treated with sac embolization for EL, as the presence of embolic material does not affect the detection of residual EL by artefact creation. Following a sac embolization, the iodinated contrast given intraoperatively remains trapped in the sac for hours or days making the assessment by CTA difficult; however, this is not a barrier for assessing residual or recurrent endoleak by CEUS.58
CEUS has the capacity of three-dimensional scanning (3D CEUS) where with the use of the new technology probes, it is possible to create a matrix array where the limitations of two-dimensional are surpassed. Gargiulo et al. studied the accuracy of 3D CEUS in 22 patients who were treated with fenestrated endovascular devices for juxta-renal aneurysm and reported correlation with the CTA in endoleak detection up to 95%.59 Abbas et al also studied the accuracy of CEUS and reported that 3D CEUS offers similar sensitivity with the CTA and commented on the ability of CEUS to provide Multi-Planar Reconstruction (MPR) and aortic segmentation therefore offering information regarding the structural integrity of the endograft as well information in real time about the haemodynamics.60
In cases of type 5 EL (endotension), sac growth is observed in the absence of endoleak. These cases are diagnosed through exclusion, however, recent data suggest that these cases represent endoleaks where the conventional imaging exams failed to visualize the persistent flow into the aneurysm sac.61,62 Millen et al presented 33 cases of endotension where CEUS identified the nature of the endoleak by analyzing the haemodynamics of the blood flow17. Acting as a problem solving tool, CEUS could be proven to be invaluable in cases where the conventional exams are not diagnostic and an endoleak of unknown origin is suspected.
Limitations of CEUS in EVAR surveillance.
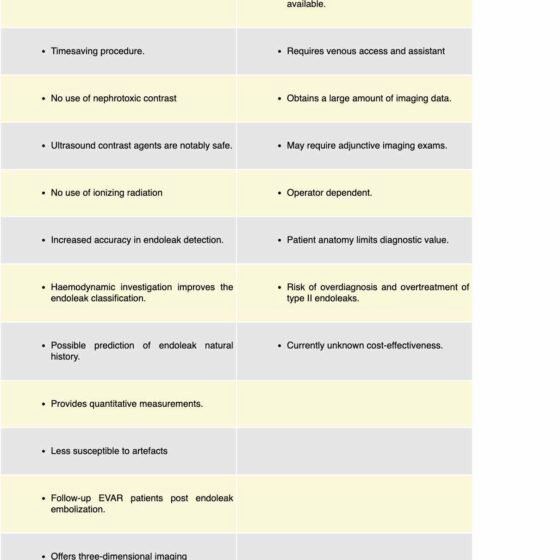
Despite the reported advantages of the utilization of CEUS in EVAR surveillance, the technique has not been broadly adopted. The required equipment is not widely available and examiners familiar with the technique are underrepresented in many centres.63 CEUS requires a peripheral venous catheter for the UCA administration and an assistant to help with the administration of the agent, making the examination somewhat cumbersome. CEUS may last for three to five minutes; however, a significant amount of data will be created following the EVAR surveillance program which will require a data storage solution increasing the overall financial cost.29
CEUS is an operator-dependent examination, where knowledge and experience can affect the outcome. The European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) recommends that CEUS examiners should have the highest level of competence and recognize that expertise in performing CEUS and interpreting the findings is gained through a lengthy process.40
Other important limitations are patient-related factors. Increased body mass index, ascites, and abdominal wall pathology can limit the acoustic window. A severely calcified plaque in the aortic wall would create an acoustic shadow, limiting the range of obtained information.53 Cases of endoleak where the feeding vessel is in an extreme posterior position may be missed.64 CEUS inspects each time a narrow area for a defined time therefore UCA administration should be repeated to complete the scanning.
Bony structures are not demonstrated by CEUS, therefore a possible endograft migration cannot be assessed due to a lack of consistent anatomical landmarks. It is not possible to measure the length of the distal and proximal sealing zones and fractures or disconnection of endograft components are not obvious unless there is also extravasation. Hence, CEUS is not a stand-alone examination. The investigation should be completed with a plain abdominal x-ray or even a CTA in cases where intervention needs to be planned in view of complications. 43 Nevertheless, Cruz et al. described that CEUS can facilitate preoperative planning by exhibiting the culprit feeding vessel in a case of type 2 endoleak, leading to a 33% reduction of the total administrated iodinated contrast.58
Most of the endoleaks that are visualized by CEUS are T2EL, which usually follow a benign course where no intervention is needed. Therefore, liberal use of CEUS could potentially lead to endoleak overdiagnosis with the risk of unnecessary interventions where the possible further complications will limit the true clinical value.
The use of UCA in EVAR surveillance has a financial impact which cannot be disregarded. The exact cost of UCA will vary per country as well as the actual cost of different imaging modalities, which can vary even between different institutions17. Faccioli et al. retrospectively studied 137 patients over six years of EVAR surveillance and reported that the cost of CEUS per case was 84.7€ compared with CTA at 157.7€. Therefore if CEUS replace CTA, a potential saving would reach 50.052,95€65. Nonetheless, Brazzelli et al. performed an extensive systematic review and economic evaluation to investigate the clinical and cost-effectiveness of different imaging strategies. By creating a Markov model and comparing five different combinations of surveillance strategies, they found that the CDU-based strategy was associated with lower cost (3799£) and higher quality-adjusted life-year (QALYs) than a CTA-based strategy (3828£) where CEUS-based strategy presented more QALYs, but at a higher cost (4709£), and became cost-effective only for high-risk patient groups66. Given the inconclusive data, further research is required to clarify the cost-effectiveness.
The evidence of CEUS accuracy in EVAR surveillance.
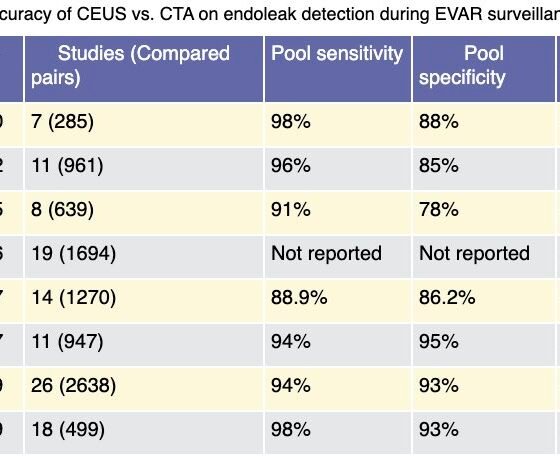
Evidence regarding the diagnostic accuracy of CEUS in EVAR surveillance is well-represented across the literature (Table 4). Despite the absence of any randomized controlled trial, non-randomized comparative and cohort studies have been reported and various systematic reviews and meta-analyses exist, analyzing the efficacy of CEUS against other imaging modalities and mainly CTA, which has been reported as the gold standard for endoleak detection. In the first meta-analysis by Mirza et al., 7 studies were analyzed and found 285 scan pairs of CEUS versus CTA and despite the heterogeneity, they found that CEUS has 0.98 polled sensitivity and 0.88 polled specificity for endoleak detection.67 Karthikesalingam et al found 961 scan pairs from 11 studies and reported similarly pooled sensitivity of 0.96 and pooled specificity of 0.85. Given CEUS as the reporting standard, CTA had a sensitivity of 70% and specificity of 98% and also reported that CEUS has higher sensitivity than CDUS (96% versus74%) in detecting any kind of endoleak; however, the reported difference was not significant in detecting type I and type III endoleaks68. Guo et al. followed a different approach and analyzed data of 3853 patients from 31 studies and by investigating three categories of scan pairs (CTA versus CDUS, CTA versus MRA and CTA versus CEUS) they measured the endoleaks that were found only by a single kind of imaging modality. From 19 studies they retrieved 1694 scan pairs of CEUS versus CTA and found that 138 endoleaks, where five of them were type II and type III endoleaks, were detected only by CEUS and conversely 51 endoleaks, where three of them were type II and type III, were found only by the CTA. Taking into consideration the fact that the reported difference in the endoleak detection was mainly related to type II endoleaks, which usually do not require intervention, the clinical value of CEUS was questioned.69 Abraha et al. in their meta-analysis found high sensitivity 0,93 [95% confidence interval (CI) 0,87-0,96] and high specificity 0,82 (95% CI 0,66-0,91) of CEUS in endoleak detection as well. To determine the clinical significance of these findings, the authors suggested that if we accept that endoleak prevalence is 22%, from a thousand EVAR patients who are investigated with CDUS, 35 endoleaks will be missed and because of type I error 47 patients will undergo an unnecessary CTA. Conversely, by using CEUS, 15 endoleaks will be missed in a total of 1000 scans.70 Kapetanios et al. included 26 studies with 2217 EVAR patients and 2217 scan pairs.71 The authors reported high pooled sensitivity and specificity of CEUS 0.94 (95% CI, 0.89-0.97) and 0.93 (95% CI, 0.89-0.96), respectively which was sustained even after the removal of the studies which had a risk of bias. By further analysis, the area under the curve (AUC) was 0.98 (95% CI, 0.93-0.99) for any kind of endoleak and 1.00 (95% CI, 0.99-1.00) for type I and type III endoleaks. In the most recent and broad meta-analysis, Karaolanis reported that the overall pooled rate of endoleak detection was 96.67% for CEUS and 92.82% for the CTA.72
The available meta-analyses have considerable limitations which should not be ignored. The sonographic technique including the kind of ultrasound device, the CTA protocol and the surveillance program varies significantly across institutions creating great heterogeneity.73 In some studies, it is not reported whether the scientists who performed the CEUS were blinded to the CTA findings and how long the interval between the CTA scan and CEUS was. The early case studies used the first generation of UCA, which had limited efficacy and in the absence of dedicated sonographic algorithms, the outcomes may have been affected. Also, some studies do not report the type of endoleak which was identified from the scan, which is an important parameter, as slow-flow endoleaks have a different clinical course to high-flow endoleaks. Finally, CTA was considered the gold standard method for endoleak detection; however, there are cases of retrograde endoleaks which were revealed by other imaging modalities and not by the CTA.
CONCLUSIONS
A growing body of evidence suggests that CEUS has a similar or even better accuracy than CTA in identifying and characterizing any type of endoleak in patients who have undergone EVAR; however, the technique has not been broadly adopted. CEUS is established as a valuable imaging modality which could reduce the number of CTA scans undertaken and be a unique solution in cases of limited options or diagnostic dilemmas. CEUS appears to have a role in EVAR surveillance programs; however, the level of its contribution needs to be defined, be it as a first-line or second-line diagnostic tool. Further, well-organized studies based on modern technology and cost analysis would guide the configuration of the best EVAR surveillance strategy.
References
- Dua A, Kuy S, Lee CJ, Upchurch GR, Desai SS. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010. J Vasc Surg. 2014;59(6):1512-1517. doi:10.1016/j.jvs.2014.01.007
- Zaiem F, Almasri J, Tello M, Prokop LJ, Chaikof EL, Murad MH. A systematic review of surveillance after endovascular aortic repair. J Vasc Surg. 2018;67(1):320-331.e37. doi:10.1016/j.jvs.2017.04.058
- Antoniou GA, Antoniou SA, Torella F. Editor’s Choice – Endovascular vs. Open Repair for Abdominal Aortic Aneurysm: Systematic Review and Meta-analysis of Updated Peri-operative and Long Term Data of Randomised Controlled Trials. Eur J Vasc Endovasc Surg. 2020;59(3):385-397. doi:10.1016/j.ejvs.2019.11.030
- Nordon IM, Karthikesalingam A, Hinchliffe RJ, Holt PJ, Loftus IM, Thompson MM. Secondary Interventions Following Endovascular Aneurysm Repair (EVAR) and the Enduring Value of Graft Surveillance. Eur J Vasc Endovasc Surg. 2010;39(5):547-554. doi:10.1016/j.ejvs.2009.11.002
- Patel R, Sweeting MJ, Powell JT, Greenhalgh RM. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388(10058):2366-2374. doi:10.1016/S0140-6736(16)31135-7
- Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg. 2019;57(1):8-93. doi:10.1016/j.ejvs.2018.09.020
- Picel AC, Kansal N. Essentials of endovascular abdominal aortic aneurysm repair imaging: Postprocedure surveillance and complications. Am J Roentgenol. 2014;203(4):W358-W372. doi:10.2214/AJR.13.11736
- Antonello M, Menegolo M, Piazza M, Bonfante L, Grego F, Frigatti P. Outcomes of endovascular aneurysm repair on renal function compared with open repair. J Vasc Surg. 2013;58(4):886-893. doi:10.1016/j.jvs.2013.02.249
- Schermerhorn ML, Buck DB, James O’Malley A, Curran T, McCallum JC, Darling J, et al. Long-term outcomes of abdominal aortic aneurysm in the medicare population. N Engl J Med. 2015;373(4):328-338. doi:10.1056/NEJMoa1405778
- Karanikola E, Dalainas I, Karaolanis G, Zografos G, Filis K. Duplex ultrasound versus computed tomography for the postoperative follow-up of endovascular abdominal aortic aneurysm repair. Where do we stand now? Int J Angiol. 2014;23(3):155-163. doi:10.1055/s-0034-1387925
- Dias N V., Riva L, Ivancev K, Resch T, Sonesson B, Malina M. Is There a Benefit of Frequent CT Follow-up After EVAR? Eur J Vasc Endovasc Surg. 2009;37(4):425-430. doi:10.1016/j.ejvs.2008.12.019
- Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2-77.e2. doi:10.1016/j.jvs.2017.10.044
- David E, Cantisani V, Grazhdani H, Di Marzo L, Venturini L, Fanelli F, et al. What is the role of contrast-enhanced ultrasound in the evaluation of the endoleak of aortic endoprostheses? A comparison between CEUS and CT on a widespread scale. J Ultrasound. 2016;19(4). doi:10.1007/s40477-016-0222-5
- Perini P, Sediri I, Midulla M, Delsart P, Mouton S, Gautier C, et al. Single-centre prospective comparison between contrast-enhanced ultrasound and computed tomography angiography after EVAR. Eur J Vasc Endovasc Surg. 2011;42(6). doi:10.1016/j.ejvs.2011.09.003
- Gürtler VM, Sommer WH, Meimarakis G, Kopp R, Weidenhagen R, Reiser MF, et al. A comparison between contrast-enhanced ultrasound imaging and multislice computed tomography in detecting and classifying endoleaks in the follow-up after endovascular aneurysm repair. J Vasc Surg. 2013;58(2):340-345. doi:10.1016/j.jvs.2013.01.039
- Bredahl KK, Taudorf M, Lönn L, Vogt KC, Sillesen H, Eiberg JP. Contrast Enhanced Ultrasound can Replace Computed Tomography Angiography for Surveillance After Endovascular Aortic Aneurysm Repair. Eur J Vasc Endovasc Surg. 2016;52(6). doi:10.1016/j.ejvs.2016.07.007
- Millen A, Canavati R, Harrison G, McWilliams RG, Wallace S, Vallabhaneni SR, et al. Defining a role for contrast-enhanced ultrasound in endovascular aneurysm repair surveillance. J Vasc Surg. 2013;58(1):18-23. doi:10.1016/j.jvs.2012.12.057
- Wei K, Skyba DM, Firschke C, Jayaweera AR, Lindner JR, Kaul S. Interactions between microbubbles and ultrasound: In vitro and in vivo observations. J Am Coll Cardiol. 1997;29(5):1081-1088. doi:10.1016/S0735-1097(97)00029-6
- Greis C. Technical aspects of contrast-enhanced ultrasound (CEUS) examinations: Tips and tricks. Clin Hemorheol Microcirc. 2014;58(1):89-95. doi:10.3233/CH-141873
- Eisenbrey JR, Sridharan A, Liu J Bin, Forsberg F. Recent experiences and advances in contrast-enhanced subharmonic ultrasound. Biomed Res Int. 2015;2015. doi:10.1155/2015/640397
- Greis C. Technology overview: SonoVue (Bracco, Milan). Eur Radiol Suppl. 2004;14(ISSUENR. 8):P11-P15. doi:10.1007/s10406-004-0076-3
- Schneider M. Characteristics of SonoVue(TM). In: Echocardiography. Vol 16. Futura Publishing Company Inc.; 1999:743-746. doi:10.1111/j.1540-8175.1999.tb00144.x
- Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsoe CP, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver – Update 2012. Ultraschall der Medizin. 2013;34(1):11-29. doi:10.1055/s-0032-1325499
- Tang C, Fang K, Guo Y, Li R, Fan X, Chen P, Chen Z, et al. Safety of Sulfur Hexafluoride Microbubbles in Sonography of Abdominal and Superficial Organs: Retrospective Analysis of 30,222 Cases. J Ultrasound Med. 2017;36(3):531-538. doi:10.7863/ultra.15.11075
- Parker JM, Weller MW, Feinstein LM, Adams RJ, Main ML, Grayburn PA, et al. Safety of ultrasound contrast agents in patients with known or suspected cardiac shunts. Am J Cardiol. 2013;112(7):1039-1045. doi:10.1016/j.amjcard.2013.05.042
- Henao EA, Hodge MD, Felkai DD, McCollum CH, Noon GP, Lin PH, et al. Contrast-enhanced Duplex surveillance after endovascular abdominal aortic aneurysm repair: Improved efficacy using a continuous infusion technique. J Vasc Surg. 2006;43(2):259-264. doi:10.1016/j.jvs.2005.09.045
- Iezzi R, Cotroneo AR, Basilico R, Simeone A, Storto ML, Bonomo L. Endoleaks after endovascular repair of abdominal aortic aneurysm: Value of CEUS. Abdom Imaging. 2010;35(1):106-114. doi:10.1007/s00261-009-9526-7
- Dietrich CF, Averkiou M, Nielsen MB, Barr RG, Burns PN, Calliada F, et al.How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int Open. 2018;4(1):E2-E15. doi:10.1055/s-0043-123931
- Barr RG. How to Develop a Contrast-Enhanced Ultrasound Program: J Ultrasound Med. 2017;36(6):1225-1240. doi:10.7863/ultra.16.09045
- Eisenbrey JR, Daecher A, Kramer MR, Forsberg F. Effects of needle and catheter size on commercially available ultrasound contrast agents. J Ultrasound Med. 2015;34(11):1961-1968. doi:10.7863/ultra.14.11008
- Gummadi, Sriharsha. A Narrative Review on Contrast-Enhanced Ultrasound in Aortic Endograft Endoleak Surveillance. Ultrasound Q. 2018;34(3):170-175. doi:10.1016/j.physbeh.2017.03.040
- Forsberg F, Liu JB, Burns PN, Merton DA, Goldberg BB. Artifacts in ultrasonic contrast agent studies. J Ultrasound Med. 1994;13(5):357-365. doi:10.7863/jum.1994.13.5.357
- Rafailidis V, Huang DY, Yusuf GT, Sidhu PS. General principles and overview of vascular contrast-enhanced ultrasonography. Ultrasonography. 2019;39(1):22-42. doi:10.14366/usg.19022
- Eckersley RJ, Chin CT, Burns PN. Optimising phase and amplitude modulation schemes for imaging microbubble contrast agents at low acoustic power. Ultrasound Med Biol. 2005;31(2):213-219. doi:10.1016/j.ultrasmedbio.2004.10.004
- Rafailidis V, Partovi S, Dikkes A, Nakamoto DA, Azar N, Staub D. Evolving clinical applications of contrast-enhanced ultrasound (CEUS) in the abdominal aorta. Cardiovasc Diagn Ther. 2018;8:S118-S130. doi:10.21037/cdt.2017.09.09
- Clevert DA, Gürtler VM, Meimarakis G, D’Anastasi M, Weidenhagen R, Reiser MF, et al. Classification of endoleaks in the follow-up after EVAR using the time-to-peak of the contrast agent in CEUS examinations. Clin Hemorheol Microcirc. 2013;55(1):183-191. doi:10.3233/CH-131701
- Dill-Macky MJ, Wilson SR, Sternbach Y, Kachura J, Lindsay T. Detecting endoleaks in aortic endografts using contrast-enhanced sonography. AJR Am J Roentgenol. 2007;188(3). doi:10.2214/AJR.05.0532
- Jung EM, Rennert J, Fellner C, Uller W, Jung W, Schreyer A, et al. Detection and Characterization of Endoleaks Following Endovascular Treatment of Abdominal Aortic Aneurysms using Contrast Harmonic Imaging (CHI) with Quantitative Perfusion Analysis (TIC) Compared to CT Angiography (CTA). Ultraschall der Medizin. 2010;31(6):564-570. doi:10.1055/s-0028-1109811
- Johnsen L, Hisdal J, Jonung T, Braaten A, Pedersen G. Contrast-enhanced ultrasound detects type II endoleaks during follow-up for endovascular aneurysm repair. In: Journal of Vascular Surgery. ; 2020. doi:10.1016/j.jvs.2020.02.020
- Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, et al. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall der Medizin. 2012;33(1):33-59. doi:10.1055/s-0031-1281676
- Raman KG, Missig-Carroll N, Richardson T, Muluk SC, Makaroun MS. Color-flow duplex ultrasound scan versus computed tomographic scan in the surveillance of endovascular aneurysm repair. In: Journal of Vascular Surgery. Vol 38. Mosby Inc.; 2003:645-651. doi:10.1016/S0741-5214(03)00909-1
- Iezzi R, Basilico R, Giancristofaro D, Pascali D, Cotroneo AR, Storto ML. Contrast-enhanced ultrasound versus color duplex ultrasound imaging in the follow-up of patients after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2009;49(3):552-560. doi:10.1016/j.jvs.2008.10.008
- Harky A, Zywicka E, Santoro G, Jullian L, Joshi M, Dimitri S. Is contrast-enhanced ultrasound (CEUS) superior to computed tomography angiography (CTA) in detection of endoleaks in post-EVAR patients? A systematic review and meta-analysis. J Ultrasound. 2019;22(1):65-75. doi:10.1007/s40477-019-00364-7
- Mitchell AM, Jones AE, Tumlin JA, Kline JA. Incidence of contrast-induced nephropathy after contrast-enhanced computed tomography in the outpatient setting. Clin J Am Soc Nephrol. 2010;5(1):4-9. doi:10.2215/CJN.05200709
- Shang Y, Xie X, Luo Y, Nie F, Luo Y, Jing X, et al. Safety findings after intravenous administration of sulfur hexafluoride microbubbles to 463,434 examinations at 24 centers. Eur Radiol. 2023;33(2). doi:10.1007/S00330-022-09108-4
- Nadolski GJ, Stavropoulos SW. Contrast alternatives for iodinated contrast allergy and renal dysfunction: Options and limitations. J Vasc Surg. 2013;57(2):593-598. doi:10.1016/j.jvs.2012.10.009
- Weerakkody RA, Walsh SR, Cousins C, Goldstone KE, Tang TY, Gaunt ME. Radiation exposure during endovascular aneurysm repair. In: British Journal of Surgery. Vol 95. Br J Surg; 2008:699-702. doi:10.1002/bjs.6229
- Jones C, Badger SA, Boyd CS, Soong C V. The impact of radiation dose exposure during endovascular aneurysm repair on patient safety. J Vasc Surg. 2010;52(2):298-302. doi:10.1016/j.jvs.2010.03.004
- McColl N, Auvinen A, Kesminiene A, Espina C, Erdmann F, de Vries E, Greinert R, Harrison J, Schüz J. European Code against Cancer 4th Edition: Ionising and non-ionising radiation and cancer. Cancer Epidemiol. 2015;39:S93-S100. doi:10.1016/j.canep.2015.03.016
- Sun C, Lin S, Zhao L, Xin S. A meta-analysis of ultrasound imaging in diagnosis of endoleak among patients after endovascular abdominal aortic aneurysm repair. Int J Clin Exp Med. 2017;10(1):1502-1512.
- Mulay S, Geraedts ACM, Koelemay MJW, Balm R, Balm R, Elshof JW, et al. Type 2 Endoleak With or Without Intervention and Survival After Endovascular Aneurysm Repair. Eur J Vasc Endovasc Surg. 2021;61(5):779-786. doi:10.1016/j.ejvs.2021.01.017
- Sidloff DA, Stather PW, Choke E, Bown MJ, Sayers RD. Type II endoleak after endovascular aneurysm repair. Br J Surg. 2013;100(10):1262-1270. doi:10.1002/bjs.9181
- Chisci E, Harris L, Guidotti A, Pecchioli A, Pigozzi C, Barbanti E, et al. Endovascular Aortic Repair Follow up Protocol Based on Contrast Enhanced Ultrasound Is Safe and Effective. Eur J Vasc Endovasc Surg. 2018;56(1):40-47. doi:10.1016/j.ejvs.2018.03.006
- Bryce Y, Schiro B, Cooper K, Ganguli S, Khayat M, Lam CK, et al. Type II endoleaks: Diagnosis and treatment algorithm. Cardiovasc Diagn Ther. 2018;8(Suppl 1):S131-S137. doi:10.21037/cdt.2017.08.06
- Chong CK, How T V., Gilling-Smith GL, Harris PL. Modeling Endoleaks and Collateral Reperfusion following Endovascular AAA Exclusion. J Endovasc Ther. 2003;10(3):424-432. doi:10.1177/152660280301000305
- Monastiriotis S, Lau I, Loh S, Ferretti J, Tassiopoulos A, Labropoulos N. Evolution of type II endoleaks based on different ultrasound-identified patterns. J Vasc Surg. 2018;67(4):1074-1081. doi:10.1016/j.jvs.2017.08.056
- Bargellini I, Napoli V, Petruzzi P, Cioni R, Vignali C, Sardella SG, et al. Type II lumbar endoleaks: Hemodynamic differentiation by contrast-enhanced ultrasound scanning and influence on aneurysm enlargement after endovascular aneurysm repair. J Vasc Surg. 2005;41(1):10-18. doi:10.1016/j.jvs.2004.10.037
- Cruz J, McGillen K, Pryor W, Esslinger D, Shin B. Novel use of contrast-enhanced ultrasound in the pretreatment planning prior to endovascular repair of endoleak after endovascular aortic aneurysm repair in a patient with chronic renal insufficiency: A case report and literature review. J Med Ultrasound. 2022;30(1):54-58. doi:10.4103/JMU.JMU_173_20
- Gargiulo M, Gallitto E, Serra C, Freyrie A, Mascoli C, Bianchini Massoni C, et al. Could four-dimensional contrast-enhanced ultrasound replace computed tomography angiography during follow up of fenestrated endografts? Results of a preliminary experience. Eur J Vasc Endovasc Surg. 2014;48(5):536-542. doi:10.1016/j.ejvs.2014.05.025
- Abbas A, Hansrani V, Sedgwick N, Ghosh J, McCollum CN. 3D contrast enhanced ultrasound for detecting endoleak following endovascular aneurysm repair (EVAR). Eur J Vasc Endovasc Surg. 2014;47(5). doi:10.1016/j.ejvs.2014.02.002
- Yoshitake A, Hachiya T, Itoh T, Kitahara H, Kasai M, Kawaguchi S, et al. Nonvisualized type III endoleak masquerading as endotension: A case report. Ann Vasc Surg. 2015;29(3):595.e15-595.e17. doi:10.1016/j.avsg.2014.10.039
- Napoli V, Bargellini I, Sardella SG, Petruzzi P, Cioni R, Vignali C, et al. Abdominal aortic aneurysm: Contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology. 2004;233(1):217-225. doi:10.1148/radiol.2331031767
- Cantisani V, Grazhdani H, Clevert DA, Iezzi R, Aiani L, Martegani A, et al. EVAR: Benefits of CEUS for monitoring stent-graft status. Eur J Radiol. 2015;84(9):1658-1665. doi:10.1016/j.ejrad.2015.07.001
- Gifford JN, Cheong HW, Teoh WC. Late-onset type I endoleak characterized by contrast enhanced ultrasound after endovascular repair of aortic aneurysm. J Clin Ultrasound. 2018;46(6):424-429. doi:10.1002/jcu.22556
- Faccioli N, Foti G, Casagranda G, Santi E, D’Onofrio M. CEUS versus CT Angiography in the follow-up of abdominal aortic endoprostheses: diagnostic accuracy and activity-based cost analysis. Radiol Medica. 2018;123(12):904-909. doi:10.1007/s11547-018-0926-z
- Brazzelli M, Hernández R, Sharma P, Robertson C, Shimonovich M, Maclennan G, et al. Contrast-enhanced ultrasound and/or colour duplex ultrasound for surveillance after endovascular abdominal aortic aneurysm repair: A systematic review and economic evaluation. Health Technol Assess (Rockv). 2018;22(72):v-220. doi:10.3310/hta22720
- Mirza TA, Karthikesalingam A, Jackson D, Walsh SR, Holt PJ, Hayes PD, et al. Duplex Ultrasound and Contrast-Enhanced Ultrasound Versus Computed Tomography for the Detection of Endoleak after EVAR: Systematic Review and Bivariate Meta-Analysis. Eur J Vasc Endovasc Surg. 2010;39(4). doi:10.1016/j.ejvs.2010.01.001
- Karthikesalingam A, Al-Jundi W, Jackson D, Boyle JR, Beard JD, Holt PJE, et al. Systematic review and meta-analysis of duplex ultrasonography, contrast-enhanced ultrasonography or computed tomography for surveillance after endovascular aneurysm repair. Br J Surg. 2012;99(11). doi:10.1002/bjs.8873
- Guo Q, Zhao J, Huang B, Yuan D, Yang Y, Zeng G, et al. A Systematic Review of Ultrasound or Magnetic Resonance Imaging Compared with Computed Tomography for Endoleak Detection and Aneurysm Diameter Measurement after Endovascular Aneurysm Repair. J Endovasc Ther. 2016;23(6):936-943. doi:10.1177/1526602816664878
- Abraha I, Luchetta ML, De Florio R, Cozzolino F, Casazza G, Duca P, et al. Ultrasonography for endoleak detection after endoluminal abdominal aortic aneurysm repair. Cochrane Database Syst Rev. 2017;2017(6). doi:10.1002/14651858.CD010296.pub2
- Kapetanios D, Kontopodis N, Mavridis D, McWilliams RG, Giannoukas AD, Antoniou GA. Meta-analysis of the accuracy of contrast-enhanced ultrasound for the detection of endoleak after endovascular aneurysm repair. J Vasc Surg. 2019;69(1). doi:10.1016/j.jvs.2018.07.044
- Karaolanis GI, Antonopoulos CN, Georgakarakos E, Lianos GD, Mitsis M, Glantzounis GK, et al. Colour Duplex and/or Contrast-Enhanced Ultrasound Compared with Computed Tomography Angiography for Endoleak Detection after Endovascular Abdominal Aortic Aneurysm Repair: A Systematic Review and Meta-Analysis. J Clin Med. 2022;11(13). doi:10.3390/jcm11133628
- Karthikesalingam A, Page AA, Pettengell C, Hinchliffe RJ, Loftus IM, Thompson MM, et al. Heterogeneity in surveillance after Endovascular Aneurysm Repair in the UK. Eur J Vasc Endovasc Surg. 2011;42(5):585-590. doi:10.1016/j.ejvs.2011.06.053
- 74. Chung J, Kordzadeh A, Prionidis I, Panayiotopoulos Y, Browne T. Contrast-enhanced ultrasound (CEUS) versus computed tomography angiography (CTA) in detection of endoleaks in post-EVAR patients. Are delayed type II endoleaks being missed? A systematic review and meta-analysis. J Ultrasound. 2015;18(2). doi:10.1007/s40477-014-0154-x