Open surgical repair of a giant iatrogenic pseudoaneurysm of the profunda femoral artery. A case report and review of the literature

| Available Online: | April, 2024 |
| Page: | 61-65 |
Author for correspondence:
Christos Argyriou, MD
Assistant Professor of Vascular Surgery, 8 Lordou Vyronos str., Alexandroupolis, Greece, 68131
E-mail: argchristos@hotmail.com, cargyriou@med.duth.gr
doi: 10.59037/a2f3nn16
ISSN 2732-7175 / 2024 Hellenic Society of Vascular and Endovascular Surgery Published by Rotonda Publications All rights reserved. https://www.heljves.com
1 Department of Vascular Surgery, Medical Faculty, “Democritus” University of Thrace, University General Hospital of Alexandroupolis, Thrace, Greece
2 Department of Interventional Radiology and Medical Imaging, Medical Faculty, “Democritus” University of Thrace, University General Hospital of Alexandroupolis, Thrace, Greece
3 Department of Cardiology, Medical Faculty, “Democritus” University of Thrace, University General Hospital of Alexandroupolis, Thrace, Greece
4 School of Medicine, Medical Faculty, “Democritus” University of Thrace, University General Hospital of Alexandroupolis, Thrace, Greece
Abstract
Full Text
References
Images
Abstract
Abstract:
We report the case of a 92-year-old male patient who suffered from a 9-cm iatrogenic pseudo-aneurysm of the left profunda femoris artery (pr.FA) and managed surgically by excision and interposition of an autologous bypass. Femoral artery (FA) pseudoaneurysms are the most common iatrogenic arterial injuries caused by FA catheterizations. In particular, they can remain clinically silent but are potentially lethal if not diagnosed or treated promptly. Endovascular or minimally invasive techniques are the predominant first-line treatment options, however in challenging cases surgical reconstruction remains the most successful definitive treatment.
Keywords: deep femoral artery peudoaneurysm, iatrogenic arterial pseudoaneurysm, surgical repair.
Full Text
INTRODUCTION
Femoral artery (FA) pseudoaneurysm results from a variety of mechanisms including blood-borne infections, trauma, injection of illegal substances, arterial access for diagnostic and endovascular procedures, closure devices, synthetic graft infections and chronic anastomotic degeneration of bypass grafts. Iatrogenic FA pseudoaneurysm is a classical complication of arterial percutaneous diagnostic angiography or interventional procedures which occurs in 0.2-2.6% of cases.1 During the recent years there is a gradual increase in their diagnosis and treatment due to the increase in endovascular catheterizations and medical interventions, both diagnostic and therapeutic.2
On the other hand, profunda FA (pr.FA) pseudoaneurysms pose a larger challenge in respect to its diagnosis and management, being more “silent” in terms of clinical examination and potentially lethal if they remain underdiagnosed. Surgery is considered the traditional revascularization option whereas endovascular and minimally invasive procedures have become attractive alternative treatment strategies and promising first-line therapeutic options.3 We describe the case of a 92-year-old male patient who suffered from a giant 9-cm in length iatrogenic pseudoaneurysm of the pr.FA and managed surgically.
CASE
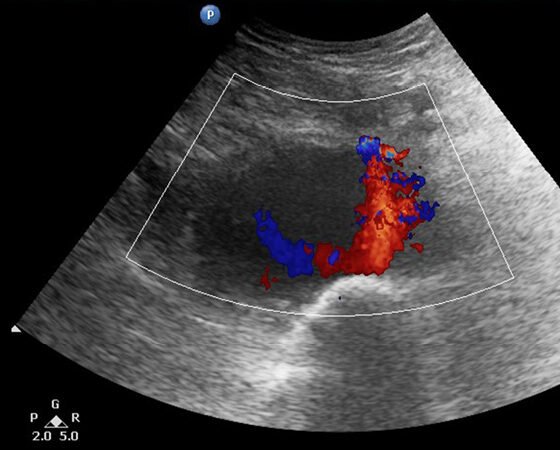
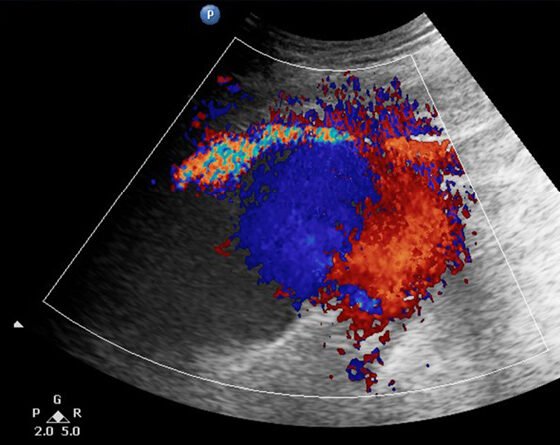
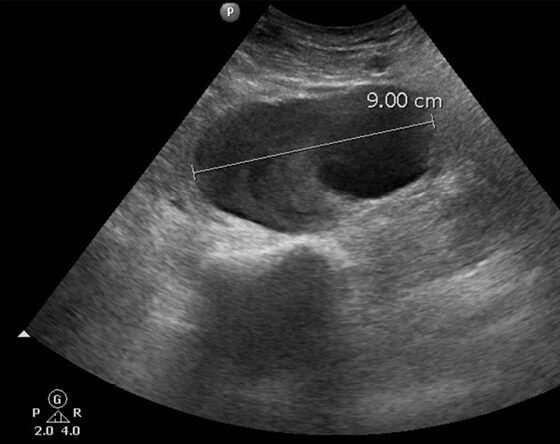
A 92-year-old male patient was admitted to the Cardiology department of our district hospital for suspected endocarditis following a 14-day period of fever of unknown etiology. Trans-esophageal echocardiogram and positive blood cultures for gram-positive cocci (staphylococcus aureus) confirmed the diagnosis. The patient had a medical history of transcatheter aortic valve replacement in a tertiary referral hospital with left femoral access three months ago and atrial fibrillation under anticoagulant treatment. Other clinical information of his past cardiac intervention could not be retrieved. At his admission to the hospital, the patient reported an increasing discomfort in his left mid-thigh, initially started as vague pain since he was at home but gradually turned into a worsening, non-remitting pain during the course of his hospitalization. At clinical examination, a pulsating painful mass in the suspected region was palpated. Ankle-brachial index was approximately 0.55 on the suspected leg due to concomitant femoropopliteal occlusive disease. Ultrasound color-duplex scanning using a 5-MHZ convex array transducer revealed a giant (9-cm in diameter, 5-cm in length) expanding pr.FA pseudoaneurysm, located approximately 5-cm distal to the femoral bifurcation, between the 2nd and 3rd perforating artery (Figures 1-3). While an endovascular option was initially considered as an attractive solution due to its minimally invasive nature, the advanced patient’s age and his current anticoagulation treatment, we opted for open surgical repair. Our treatment strategy was principally technically defined due to the broad base without neck expanding pr.FA pseudoaneurysm and also clinically driven to minimize the possibility of establishing permanent neuropathy by surgical evacuation of the hematoma causing compressive symptoms. An additional significant parameter in decision making was the lack of an established diagnosis by the time of intervention, with possible diagnoses including iatrogenic or infected pseudoaneurysm. In the latter case we would prefer to avoid placement of endovascular materials in infected areas.
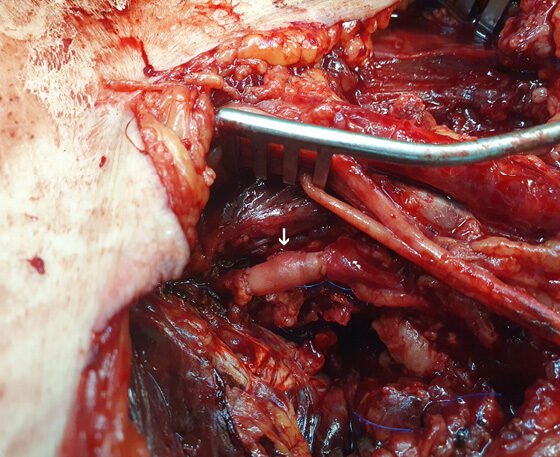
Therefore, through an extended anteromedial left groin surgical approach exposing the femoral bifurcation and its branches, the culprit branch of the pr.FA pseudoaneurysm was dissected and excised while reconstruction was completed by interposition of an autologous bypass using segment of the distal ipsilateral greater saphenous vein (Figure 4). Perioperatively, 3 units of packed red blood cells were transfused. The patient had an uneventful recovery, having a mild wound lymphorrhea treated conservatively with elastic compression, completely resolving at 3 months post-operatively. Pseudoaneurysm and arterial wall microbiology cultures were negative for any infectious organism. Follow-up at 6 months revealed no complications.
DISCUSSION
The common FA is the most common access site for endovascular procedures, and it is therefore essential for interventionists to know how to recognize and manage iatrogenic complications associated with this type of access. Pseudoaneurysms are reported to occur in 1% of diagnostic arteriograms and up to 8% of therapeutic endovascular interventions.4 The most frequent complications range from 0.5% to 8% and include hematoma, uncontrollable groin and/ or retroperitoneal bleeding, Pseudoaneurysm formation, arteriovenous fistula, and in situ arterial dissection with or without associated thrombosis.5 Less frequent complications include distal embolization, nerve damage, abscess, and lymphocele.5 In recent years, a number of percutaneous closure devices have been developed, with the hope of reducing the frequency of local complications and allowing early patient mobilization post endovascular intervention. Large clinical trials have indeed demonstrated patients’ preference for the use of these closure devices compared to the long bedrest associated with the more traditional method of manual compression. However, these studies failed to demonstrate a reduction in local complications associated with the use of these devices.6
Coronary angiography, angioplasty and stenting are recognized risk factors for FA pseudoaneurysm formation.7 Procedural factors, which mostly influence risk of complications include use of a sheath size greater than 8 Fr. and faulty puncture technique, either puncture above the inguinal ligament or puncture of the superficial FA or pr.FA.8,9 The latter occurs more frequently if the origin of pr.FA originates behind or even above the inguinal ligament, an anatomical variation observed in 25% of cases.10 Patient-specific risk factors include arterial hypertension, female gender, coagulopathy, severe peripheral arterial disease, hostile groin anatomy, high femoral bifurcation, nonflexible hip, advanced age, obesity, erythematous skin, intertrigo and anticoagulation, antiplatelet or fibrinolytic medication.11
Endovascular repair of femoral arterial access complications is considered an attractive solution in patients who cannot tolerate vascular reconstruction and bleeding due to advanced cardiovascular disease.12 The number of percutaneous endovascular interventions performed worldwide has been growing rapidly due to important technological advances, improved long-term clinical outcomes, and, also, the lower morbidity associated with these procedures compared with traditional surgical techniques.13 Endovascular procedures can be performed under local anesthesia, are well tolerated by the patient, and are associated with a short hospitalization time. Nitinol stent technology allows for safe stent and stent-graft extension at the common FA level, due to increased resistance to external compression and bending stress.14 Thalhammer et al included 26 patients after repair of iatrogenic pseudoaneurysms of the femoral bifurcation, reporting high technical and clinical success, concluding that stent-graft placement is an effective, low-risk procedure, especially in high-risk patients.12 Despite absence of long-term results for the covered stent technology in the literature, relative contraindications for the endovascular approach could include young patients who are good candidates for open surgery, extreme elongation and tortuosity of the femoral vessels, short femoral bifurcation and increased cost of the device.13
Ultrasound-guided compression is an effective treatment for FA pseudoaneurysms, with success rates of ranging from 70% to 90% in patients without anticoagulation therapy.15 Nevertheless, this method has significant limitations. It is painful and time-consuming, with suboptimal results in patients who are obese and under anticoagulant treatment.16 As an alternative, direct percutaneous embolization of the pseudoaneurysm sac with different embolic materials has been reported. Thrombin is the most popular embolic agent used for percutaneous occlusion of FA pseudoaneurysms because it causes fast and efficient thrombosis in the aneurysmal sac without filling it with foreign material.17 However, some complications may occur with this agent, including allergic reaction and distal thrombosis of the parent artery by leakage through the pseudoaneurysm neck.18 Kurzawski et al. prospectively studied 353 patients treated by thrombin injection and reported 53 (15%) arterial microembolizations and 1 (0.3%) pulmonary embolism in a case of concomitant arterial-venous fistula.19 Comparing these two methods, the only randomized clinical trial existing in the literature concluded that percutaneous thrombin injection appeared to be more effective than ultrasound-guided compression in achieving primary thrombosis of a pseudoaneurysm, although other case series in the literature have reported higher pseudoaneurysm sac thrombosis rates with compression compared to previous case series.14,20
N-butyl-2 cyanocrylate glue is a potential alternative to thrombin, initially described or treating arterio-venous malformations amenable to endovascular interventions.21 However, its main drawback is the risk of direct percutaneous distal embolization due to escape of the material from the pseudoaneurysmal sac before it is completely polymerized.22 For this reason, Griviau et al described the technique of inflating an angioplasty balloon at the orifice of the pseudoaneurysm during glue injection and to avoid blood backflow and reflux of glue into the native artery.23 Potentially less effective method for reducing the risk of distal ischemia or recurrence of the pseudoaneurysm is manual effective methods to decrease the risk of distal embolization is manual compression of the either neck or the sac of the pseudoaneurysm by ultrasound until no sac flow is observed in the pseudoaneurysm, followed by glue injection. However, these techniques are more technically challenging to identify the no-flow pseudoaneurysm after compression by ultrasound, especially if the patient is obese or the hematoma is large.24,25
In the era of endovascular evolution, surgical treatment of FA pseudoaneurysms is less common but can be life-saving, especially when other modes of intervention are more likely to fail or are contraindicated. Major indications that favor surgical management over endovascular management are infected pseudoaneurysms, rapid sac expansion, skin necrosis and compressive symptoms such as neuropathy, claudication and critical limb ischemia.3 Among the surgical solutions, interposition bypass, saphenous vein patch angioplasty and vessel ligation are included. On the other hand, surgical reconstruction leads to increased local complications and prolonged hospitalization. Although there are no large clinical series or comparative studies existing in the literature, the implementation of endovascular or minimally invasive techniques could theoretically provide an advantage in terms of morbidity and mortality compared to open surgery. This avoids the need for a general anesthetic in a group of patients with significant comorbidities who cannot tolerate vascular general reconstruction with concomitant blood loss.14
In our patient, obesity, a small-neck with a large diameter pr.FA pseudoaneurysm surrounded by an extended hematoma and the crucial loss of the only outflow vessel (pr.FA) in the leg applying risky procedures were unsuitable factors for endovascular or minimally invasive strategy. Further selection criteria for open surgical reconstruction were the rapidly expanding pseudoaneurysm from the continuous intrasac bleeding and worsening neuropathy due to local compression symptoms of the femoral nerve. In this regard, resolution of the symptoms would be improved only after surgical decompression of the hematoma, reducing also the risk of a future infected hematoma in the groin region.
Conclusion
Femoral PAs are relatively common iatrogenic injuries following therapeutic and diagnostic catheterization procedures. In particular, pr.FA pseudoaneurysms are clinically silent but potentially lethal, especially if remain underdiagnosed or left untreated. Although endovascular or minimally invasive techniques have been evolving and transforming into the gold-standard treatment option, surgical reconstruction still remains the sine qua non of vascular repair, especially in selected cases not amenable to endovascular solutions.
The Authors state that
(1) there has been no duplicate publication or submission of any part of the revised work;
(2) all have read and approved the revised manuscript; and
(3) This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector
(4) there is no conflict of interest within the manuscript
(5) Informed consent has been obtained from the patient for publication of the case report and accompanying images.
References
- Katzenschlager R, Ugurluoglu A, Ahmadi A et al. Incidence of pseudoaneurysm after diagnostic and therapeutic angiography. Radiology 1995;195(2):463-466.
- Moini M, Rasouli MR, Rayatzadeh H et al. Management of femoral artery pseudo-aneurysms in Iran: a single centre report of 50 cases. Acta Chir Belg 2008;108(2):226-230.
- Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation 2007;115(20):2666-2674.
- Etemad-Rezai R, Peck DJ. Ultrasound-guided thrombin injection of femoral artery pseudoaneurysms. Can Assoc Radiol J 2003;54(2):118-120.
- Bourassa MG, Noble J. Complication rate of coronary arteriography. A review of 5250 cases studied by a percutaneous femoral technique. Circulation 1976;53(1):106-114.
- Koreny M, Riedmüller E, Nikfardjam Me et al. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA 2004;291(3):350-357.
- Kresowik TF, Khoury MD, Miller BV et al. A prospective study of the incidence and natural history of femoral vascular complications after percutaneous transluminal coronary angioplasty. J Vasc Surg 1991;13(2):328-333.
- Muller DW, Shamir KJ, Ellis SG et al. Peripheral vascular complications after conventional and complex percutaneous coronary interventional procedures. Am J Cardiol 1992;69(1):63-68.
- Davis C, VanRiper S, Longstreet J et al. Vascular complications of coronary interventions. Heart Lung 1997;26(2):118-127.
- Haimovici H. Vascular Surgery: principles and techniques. Norwalk, 2nd ed. Appleton-Century-Crofts, 1984:537-546.
- Ates M, Sahin S, Konuralp C et al. Evaluation of risk factors associated with femoral pseudoaneurysms after cardiac catheterization. J Vasc Surg 2006;43(3):520-524.
- Thalhammer C, Kirchherr AS, Uhlich F et al. Postcatheterization pseudoaneurysms and arteriovenous fistulas: repair with percutaneous implantation of endovascular covered stents. Radiology 2000;214(1):127-131.
- Tsetis D. Endovascular treatment of complications of femoral arterial access. Cardiovasc Intervent Radiol 2010;33(3):457-468.
- Morgan R, Belli AM. Current treatment methods for postcatheterization pseudoaneurysms. J VascIntervRadiol 2003;14(6):697-710.
- Lange P, Houe T, Helgstrand UJ. The efficacy of ultrasound-guided compression of iatrogenic femoral pseudo-aneurysms. Eur J VascEndovasc Surg 2001;21(3):248-250.
- Cox GS, Young JR, Gray BR et al. Ultrasound-guided compression repair of postcatheterization pseudoaneurysms: results of treatment in one hundred cases. J Vasc Surg 1994;19(4):683-686.
- Ergun O, Çeltikçi P, Güneş Tatar İ et al. Percutaneous thrombin injection treatment of a femoral artery pseudoaneurysm with simultaneous arterial balloon occlusion: Case report and review of the literature. Turk Kardiyol Dern Ars 2016;44(8):684-689.
- Loffroy R. N-Butyl Cyanoacrylate Glue: The Best Hemostatic Embolic Agent for Patients with Acute Arterial Bleeding. Cardiovasc Intervent Radiol 2017;40(8):1290-1291.
- Kurzawski J, Sadowski M, Janion-Sadowska A. Complications of percutaneous thrombin injection in patients with postcatheterization femoral pseudoaneurysm. J Clin Ultrasound 2016;44(3):188-195.
- Lönn L, Olmarker A, Geterud K et al. Treatment of femoral pseudoaneurysms. Percutaneous US-guided thrombin injection versus US-guided compression. Acta Radiol 2002;43(4):396-400.
- Parihar A, Tomar S, Phadke RV. Direct sac puncture and glue embolization of intraosseous AVM of the maxilla. Int J Oral Maxillofac Surg 2011;40(7):749-752.
- Le TD, Than VS, Nguyen MD. Percutaneous balloon-assisted ultrasound-guided direct glue embolization of deep femoral artery pseudoaneurysm rupture. Radiol Case Rep 2020;16(3):425-429.
- Griviau L, Chevallier O, Marcelin C et al. Percutaneous ultrasound-guided balloon-assisted embolization of iatrogenic femoral artery pseudoaneurysms with Glubran®2 cyanoacrylate glue: safety, efficacy and outcomes. Quant Imaging Med Surg 2018;8(8):796-803.
- Del Corso A, Vergaro G. Percutaneous treatment of iatrogenic pseudoaneurysms by cyanoacrylate-based wall-gluing. Cardiovasc Intervent Radiol 2013;36(3):669-675.
- Aytekin C, Firat A, Yildirim E et al. Ultrasound-guided glue injection as alternative treatment of femoral pseudoaneurysms. Cardiovasc Intervent Radiol 2004;27(6):612-615.